Amyloid beta
| Amyloid beta peptide (beta-APP) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 A partially folded structure of amyloid beta(1 40) in an aqueous environment (pdb 2lfm)[1] | |||||||||
| Identifiers | |||||||||
| Symbol | APP | ||||||||
| Pfam | PF03494 | ||||||||
| InterPro | IPR013803 | ||||||||
| SCOP2 | 2lfm / SCOPe / SUPFAM | ||||||||
| TCDB | 1.C.50 | ||||||||
| OPM superfamily | 304 | ||||||||
| OPM protein | 2y3k | ||||||||
| Membranome | 45 | ||||||||
| |||||||||
| amyloid beta (A4) precursor protein (peptidase nexin-II, Alzheimer disease) | |||||||
|---|---|---|---|---|---|---|---|
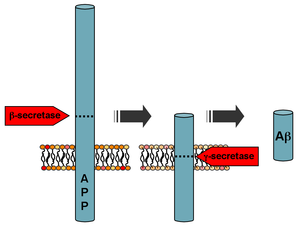 Processing of the amyloid precursor protein | |||||||
| Identifiers | |||||||
| Symbol | APP | ||||||
| Alt. symbols | AD1 | ||||||
| NCBI gene | 351 | ||||||
| HGNC | 620 | ||||||
| OMIM | 104760 | ||||||
| RefSeq | NM_000484 | ||||||
| UniProt | P05067 | ||||||
| Other data | |||||||
| Locus | Chr. 21 q21.2 | ||||||
| |||||||
Amyloid beta (Aβ, Abeta or beta-amyloid) denotes peptides of 36–43 amino acids that are the main component of the amyloid plaques found in the brains of people with Alzheimer's disease.[2] The peptides derive from the amyloid-beta precursor protein (APP), which is cleaved by beta secretase and gamma secretase to yield Aβ in a cholesterol-dependent process and substrate presentation.[3] Both neurons and oligodendrocytes produce and release Aβ in the brain, contributing to formation of amyloid plaques.[4] Aβ molecules can aggregate to form flexible soluble oligomers which may exist in several forms. It is now believed that certain misfolded oligomers (known as "seeds") can induce other Aβ molecules to also take the misfolded oligomeric form, leading to a chain reaction akin to a prion infection. The oligomers are toxic to nerve cells.[5] The other protein implicated in Alzheimer's disease, tau protein, also forms such prion-like misfolded oligomers, and there is some evidence that misfolded Aβ can induce tau to misfold.[6][7]
A study has suggested that APP and its amyloid potential is of ancient origins, dating as far back as early deuterostomes.[8]
Normal function
[edit]The normal function of Aβ is not yet known.[9] Though some animal studies have shown that the absence of Aβ does not lead to any obvious loss of physiological function,[10][11] several potential activities have been discovered for Aβ, including activation of kinase enzymes,[12][13] protection against oxidative stress,[14][15] regulation of cholesterol transport,[16][17] functioning as a transcription factor,[18][19] and anti-microbial activity (potentially associated with Aβ's pro-inflammatory activity).[20][21][22]
The glymphatic system clears metabolic waste from the mammalian brain, and in particular amyloid beta.[23] A number of proteases have been implicated by both genetic and biochemical studies as being responsible for the recognition and degradation of amyloid beta; these include insulin degrading enzyme[24] and presequence protease.[25] The rate of removal is significantly increased during sleep.[26] However, the significance of the glymphatic system in Aβ clearance in Alzheimer's disease is unknown.[27]
Disease associations
[edit]Aβ is the main component of amyloid plaques, extracellular deposits found in the brains of people with Alzheimer's disease.[28] Aβ can also form the deposits that line cerebral blood vessels in cerebral amyloid angiopathy. The plaques are composed of a tangle of Aβ oligomers[29] and regularly ordered aggregates called amyloid fibrils,[30] a protein fold shared by other peptides such as the prions associated with protein misfolding disease, also known as proteinopathy.
Alzheimer's disease
[edit]Research suggests that soluble oligomeric forms of the amyloid beta may be causative agents in the development of Alzheimer's disease.[31] It is generally believed that Aβ oligomers are the most toxic.[32] Several genetic, cell biology, biochemical and animal studies using experimental models support the concept that Aβ plays a central role in the development of Alzheimer's disease pathology.[33][34]
Brain Aβ is elevated in people with sporadic Alzheimer's disease. Aβ is the main constituent of brain parenchymal and vascular amyloid; it contributes to cerebrovascular lesions and is neurotoxic.[33][34][35] It is unresolved how Aβ accumulates in the central nervous system and subsequently initiates the disease of cells. Significant efforts have been focused on the mechanisms responsible for Aβ production, including the proteolytic enzymes gamma- and β-secretases which generate Aβ from its precursor protein, APP (amyloid precursor protein).[36][37] Aβ circulates in plasma, cerebrospinal fluid (CSF) and brain interstitial fluid (ISF) mainly as soluble Aβ40.[33][38] Amyloid plaques contain both Aβ40 and Aβ42,[39] while vascular amyloid is predominantly the shorter Aβ40. Several sequences of Aβ were found in both lesions.[40][41]
Increases in either total Aβ levels or the relative concentration of both Aβ40 and Aβ42 (where the former is more concentrated in cerebrovascular plaques and the latter in neuritic plaques)[42] have been implicated in the pathogenesis of both familial and sporadic Alzheimer's disease. Due to its more hydrophobic nature, the Aβ42 is the most amyloidogenic form of the peptide. However the central sequence KLVFFAE is known to form amyloid on its own, and probably forms the core of the fibril.[citation needed] One study further correlated Aβ42 levels in the brain not only with onset of Alzheimer's disease, but also reduced cerebrospinal fluid pressure, suggesting that a build-up or inability to clear Aβ42 fragments may play a role into the pathology.[43]
The "amyloid hypothesis" — that the plaques are responsible for the pathology of Alzheimer's disease — is accepted by the majority of researchers, but is not conclusively established. An alternative hypothesis is that amyloid oligomers rather than plaques are responsible for the disease.[32][44]
Cancer
[edit]While Aβ has been implicated in cancer development, prompting studies on a variety of cancers to elucidate the nature of its possible effects, results are largely inconclusive. Aβ levels have been assessed in relation to a number of cancers, including esophageal, colorectal, lung, and hepatic, in response to observed reductions in risk for developing Alzheimer's disease in survivors of these cancers. All cancers were shown to be associated positively with increased Aβ levels, particularly hepatic cancers.[45] This direction of association however has not yet been established. Studies focusing on human breast cancer cell lines have further demonstrated that these cancerous cells display an increased level of expression of amyloid precursor protein.[46]
Down syndrome
[edit]Adults with Down syndrome had accumulation of amyloid in association with evidence of Alzheimer's disease, including declines in cognitive functioning, memory, fine motor movements, executive functioning, and visuospatial skills.[47]
Formation
[edit]Aβ is formed after sequential cleavage of the amyloid precursor protein (APP), a transmembrane glycoprotein of undetermined function. APP can be cleaved by the proteolytic enzymes α-, β- and γ-secretase; Aβ protein is generated by successive action of the β and γ secretases. The γ secretase, which produces the C-terminal end of the Aβ peptide, cleaves within the transmembrane region of APP and can generate a number of isoforms of 30–51 amino acid residues in length.[48] The most common isoforms are Aβ40 and Aβ42; the longer form is typically produced by cleavage that occurs in the endoplasmic reticulum, while the shorter form is produced by cleavage in the trans-Golgi network.[49]
Genetics
[edit]Autosomal-dominant mutations in APP cause hereditary early-onset Alzheimer's disease (familial AD, fAD). This form of AD accounts for no more than 10% of all cases, and the vast majority of AD is not accompanied by such mutations.[50] However, familial Alzheimer's disease is likely to result from altered proteolytic processing. This is evidenced by the fact that many mutations that lead to fAD occur near γ-secretase cleavage sites on APP.[51] One of the most common mutations causing fAD, London Mutation, occurs at codon 717 of the APP gene,[52][53] and results in a valine to isoleucine amino acid substitution. Histochemical analysis of the APP V717I mutation has revealed extensive Aβ pathology throughout neuroaxis as well as widespread cerebral amyloid angiopathy (CAA).[54]
The gene for the amyloid precursor protein is located on chromosome 21, and accordingly people with Down syndrome have a very high incidence of Alzheimer's disease.[55]
Structure and toxicity
[edit]Amyloid beta is commonly thought to be intrinsically unstructured, meaning that in solution it does not acquire a unique tertiary fold but rather populates a set of structures. As such, it cannot be crystallized and most structural knowledge on amyloid beta comes from NMR and molecular dynamics. Early NMR-derived models of a 26-aminoacid polypeptide from amyloid beta (Aβ 10–35) show a collapsed coil structure devoid of significant secondary structure content.[56] However, the most recent (2012) NMR structure of (Aβ 1-40) has significant secondary and tertiary structure.[1] Replica exchange molecular dynamics studies suggested that amyloid beta can indeed populate multiple discrete structural states;[57] more recent studies identified a multiplicity of discrete conformational clusters by statistical analysis.[58] By NMR-guided simulations, amyloid beta 1-40 and amyloid beta 1-42 also seem to feature highly different conformational states,[59] with the C-terminus of amyloid beta 1-42 being more structured than that of the 1-40 fragment.
Low-temperature and low-salt conditions allowed to isolate pentameric disc-shaped oligomers devoid of beta structure.[60] In contrast, soluble oligomers prepared in the presence of detergents seem to feature substantial beta sheet content with mixed parallel and antiparallel character, different from fibrils;[61] computational studies suggest an antiparallel beta-turn-beta motif instead for membrane-embedded oligomers.[62]
Immunotherapy research
[edit]Immunotherapy may stimulate the host immune system to recognize and attack Aβ, or provide antibodies that either prevent plaque deposition or enhance clearance of plaques or Aβ oligomers. Oligomerization is a chemical process that converts individual molecules into a chain consisting of a finite number of molecules. Prevention of oligomerization of Aβ has been exemplified by active or passive Aβ immunization. In this process antibodies to Aβ are used to decrease cerebral plaque levels. This is accomplished by promoting microglial clearance and/or redistributing the peptide from the brain to systemic circulation. Antibodies that target Aβ and were tested in clinical trials included aducanumab, bapineuzumab, crenezumab, gantenerumab, lecanemab, and solanezumab.[63][64]
Measuring amyloid beta
[edit]
Imaging compounds, notably Pittsburgh compound B, (6-OH-BTA-1, a thioflavin), can selectively bind to amyloid beta in vitro and in vivo. This technique, combined with PET imaging, is used to image areas of plaque deposits in those with Alzheimer's.[65]
Post mortem or in tissue biopsies
[edit]Amyloid beta can be measured semiquantitatively with immunostaining, which also allows one to determine location. Amyloid beta may be primarily vascular, as in cerebral amyloid angiopathy, or in amyloid plaques in white matter.[66]
One sensitive method is ELISA which is an immunosorbent assay which utilizes a pair of antibodies that recognize amyloid beta.[67][68]
Atomic force microscopy, which can visualize nanoscale molecular surfaces, can be used to determine the aggregation state of amyloid beta in vitro.[69]
Vibrational microspectroscopy is a label-free method that measures the vibration of molecules in tissue samples.[70] Amyloid proteins like Aβ can be detected with this technique because of their high content of β-sheet structures.[71] Recently, the formation of Aβ fibrils was resolved in different plaque-types in Alzheimer's disease, indicating that plaques transit different stages in their development.[29]
Dual polarisation interferometry is an optical technique which can measure early stages of aggregation by measuring the molecular size and densities as the fibrils elongate.[72][73] These aggregate processes can also be studied on lipid bilayer constructs.[74]
See also
[edit]- TPM21
- Sylvain Lesné – Aβ*56
References
[edit]- ^ a b Vivekanandan S, Brender JR, Lee SY, Ramamoorthy A (July 2011). "A partially folded structure of amyloid-beta(1-40) in an aqueous environment". Biochemical and Biophysical Research Communications. 411 (2): 312–316. doi:10.1016/j.bbrc.2011.06.133. PMC 3148408. PMID 21726530.
- ^ Hamley IW (October 2012). "The amyloid beta peptide: a chemist's perspective. Role in Alzheimer's and fibrillization" (PDF). Chemical Reviews. 112 (10): 5147–5192. doi:10.1021/cr3000994. PMID 22813427.
- ^ Wang H, Kulas JA, Wang C, Holtzman DM, Ferris HA, Hansen SB (August 2021). "Regulation of beta-amyloid production in neurons by astrocyte-derived cholesterol". Proceedings of the National Academy of Sciences of the United States of America. 118 (33): e2102191118. Bibcode:2021PNAS..11802191W. doi:10.1073/pnas.2102191118. PMC 8379952. PMID 34385305.
- ^ Rajani RM, Ellingford R, Hellmuth M, Harris SS, Taso OS, Graykowski D, et al. (July 2024). "Selective suppression of oligodendrocyte-derived amyloid beta rescues neuronal dysfunction in Alzheimer's disease". PLOS Biology. 22 (7): e3002727. doi:10.1371/journal.pbio.3002727. PMC 11265669. PMID 39042667.
- ^ Haass C, Selkoe DJ (February 2007). "Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide". Nature Reviews. Molecular Cell Biology. 8 (2): 101–112. doi:10.1038/nrm2101. PMID 17245412. S2CID 32991755.
- ^ Nussbaum JM, Seward ME, Bloom GS (Jan–Feb 2013). "Alzheimer disease: a tale of two prions". Prion. 7 (1): 14–19. doi:10.4161/pri.22118. PMC 3609044. PMID 22965142.
- ^ Pulawski W, Ghoshdastider U, Andrisano V, Filipek S (April 2012). "Ubiquitous amyloids". Applied Biochemistry and Biotechnology. 166 (7): 1626–1643. doi:10.1007/s12010-012-9549-3. PMC 3324686. PMID 22350870.
- ^ Tharp WG, Sarkar IN (April 2013). "Origins of amyloid-β". BMC Genomics. 14 (1): 290. doi:10.1186/1471-2164-14-290. PMC 3660159. PMID 23627794.
- ^ Hiltunen M, van Groen T, Jolkkonen J (2009). "Functional roles of amyloid-beta protein precursor and amyloid-beta peptides: evidence from experimental studies". Journal of Alzheimer's Disease. 18 (2): 401–412. doi:10.3233/JAD-2009-1154. PMID 19584429.
- ^ Sadigh-Eteghad S, Talebi M, Farhoudi M, EJ Golzari S, Sabermarouf B, Mahmoudi J (2014). "Beta-amyloid exhibits antagonistic effects on alpha 7 nicotinic acetylcholine receptors in orchestrated manner". Journal of Medical Hypotheses and Ideas. 8 (2): 48–52. doi:10.1016/j.jmhi.2014.01.001.
- ^ Luo Y, Bolon B, Damore MA, Fitzpatrick D, Liu H, Zhang J, et al. (October 2003). "BACE1 (beta-secretase) knockout mice do not acquire compensatory gene expression changes or develop neural lesions over time". Neurobiology of Disease. 14 (1): 81–88. doi:10.1016/S0969-9961(03)00104-9. PMID 13678669. S2CID 8367440.
- ^ Bogoyevitch MA, Boehm I, Oakley A, Ketterman AJ, Barr RK (March 2004). "Targeting the JNK MAPK cascade for inhibition: basic science and therapeutic potential". Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics. 1697 (1–2): 89–101. doi:10.1016/j.bbapap.2003.11.016. PMID 15023353.
- ^ Tabaton M, Zhu X, Perry G, Smith MA, Giliberto L (January 2010). "Signaling effect of amyloid-beta(42) on the processing of AbetaPP". Experimental Neurology. 221 (1): 18–25. doi:10.1016/j.expneurol.2009.09.002. PMC 2812589. PMID 19747481.
- ^ Zou K, Gong JS, Yanagisawa K, Michikawa M (June 2002). "A novel function of monomeric amyloid beta-protein serving as an antioxidant molecule against metal-induced oxidative damage". The Journal of Neuroscience. 22 (12): 4833–4841. doi:10.1523/JNEUROSCI.22-12-04833.2002. PMC 6757724. PMID 12077180.
- ^ Baruch-Suchodolsky R, Fischer B (May 2009). "Abeta40, either soluble or aggregated, is a remarkably potent antioxidant in cell-free oxidative systems". Biochemistry. 48 (20): 4354–4370. doi:10.1021/bi802361k. PMID 19320465.
- ^ Yao ZX, Papadopoulos V (October 2002). "Function of beta-amyloid in cholesterol transport: a lead to neurotoxicity". FASEB Journal. 16 (12): 1677–1679. doi:10.1096/fj.02-0285fje. PMID 12206998. S2CID 17813857.
- ^ Igbavboa U, Sun GY, Weisman GA, He Y, Wood WG (August 2009). "Amyloid beta-protein stimulates trafficking of cholesterol and caveolin-1 from the plasma membrane to the Golgi complex in mouse primary astrocytes". Neuroscience. 162 (2): 328–338. doi:10.1016/j.neuroscience.2009.04.049. PMC 3083247. PMID 19401218.
- ^ Maloney B, Lahiri DK (November 2011). "The Alzheimer's amyloid β-peptide (Aβ) binds a specific DNA Aβ-interacting domain (AβID) in the APP, BACE1, and APOE promoters in a sequence-specific manner: characterizing a new regulatory motif". Gene. 488 (1–2): 1–12. doi:10.1016/j.gene.2011.06.004. PMC 3381326. PMID 21699964.
- ^ Bailey JA, Maloney B, Ge YW, Lahiri DK (November 2011). "Functional activity of the novel Alzheimer's amyloid β-peptide interacting domain (AβID) in the APP and BACE1 promoter sequences and implications in activating apoptotic genes and in amyloidogenesis". Gene. 488 (1–2): 13–22. doi:10.1016/j.gene.2011.06.017. PMC 3372404. PMID 21708232.
- ^ Kagan BL, Jang H, Capone R, Teran Arce F, Ramachandran S, Lal R, et al. (April 2012). "Antimicrobial properties of amyloid peptides". Molecular Pharmaceutics. 9 (4): 708–717. doi:10.1021/mp200419b. PMC 3297685. PMID 22081976.
- ^ Schluesener HJ, Su Y, Ebrahimi A, Pouladsaz D (June 2012). "Antimicrobial peptides in the brain: neuropeptides and amyloid". Frontiers in Bioscience. 4 (4): 1375–1380. doi:10.2741/S339. PMID 22652879.
- ^ Li H, Liu CC, Zheng H, Huang TY (2018). "Amyloid, tau, pathogen infection and antimicrobial protection in Alzheimer's disease -conformist, nonconformist, and realistic prospects for AD pathogenesis". Translational Neurodegeneration. 7: 34. doi:10.1186/s40035-018-0139-3. PMC 6306008. PMID 30603085.
- ^ Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. (August 2012). "A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β". Science Translational Medicine. 4 (147): 147ra111. doi:10.1126/scitranslmed.3003748. PMC 3551275. PMID 22896675.
- ^ Shen Y, Joachimiak A, Rosner MR, Tang WJ (October 2006). "Structures of human insulin-degrading enzyme reveal a new substrate recognition mechanism". Nature. 443 (7113): 870–874. Bibcode:2006Natur.443..870S. doi:10.1038/nature05143. PMC 3366509. PMID 17051221.
- ^ King JV, Liang WG, Scherpelz KP, Schilling AB, Meredith SC, Tang WJ (July 2014). "Molecular basis of substrate recognition and degradation by human presequence protease". Structure. 22 (7): 996–1007. doi:10.1016/j.str.2014.05.003. PMC 4128088. PMID 24931469.
- ^ Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, et al. (October 2013). "Sleep drives metabolite clearance from the adult brain". Science. 342 (6156): 373–377. Bibcode:2013Sci...342..373X. doi:10.1126/science.1241224. PMC 3880190. PMID 24136970.
- ^ Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, et al. (August 2015). "Clearance systems in the brain-implications for Alzheimer disease". Nature Reviews. Neurology. 11 (8): 457–470. doi:10.1038/nrneurol.2015.119. PMC 4694579. PMID 26195256.
- ^ Sadigh-Eteghad S, Sabermarouf B, Majdi A, Talebi M, Farhoudi M, Mahmoudi J (2014). "Amyloid-beta: a crucial factor in Alzheimer's disease". Medical Principles and Practice. 24 (1): 1–10. doi:10.1159/000369101. PMC 5588216. PMID 25471398.
- ^ a b Röhr D, Boon BD, Schuler M, Kremer K, Hoozemans JJ, Bouwman FH, et al. (December 2020). "Label-free vibrational imaging of different Aβ plaque types in Alzheimer's disease reveals sequential events in plaque development". Acta Neuropathologica Communications. 8 (1): 222. doi:10.1186/s40478-020-01091-5. PMC 7733282. PMID 33308303.
- ^ Parker MH, Reitz AB (2000). "Assembly of β-Amyloid Aggregates at the Molecular Level". Chemtracts-Organic Chemistry. 13 (1): 51–56.
- ^ Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, et al. (August 2008). "Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory". Nature Medicine. 14 (8): 837–842. doi:10.1038/nm1782. PMC 2772133. PMID 18568035.
- ^ a b Zhao LN, Long HW, Mu Y, Chew LY (2012). "The toxicity of amyloid β oligomers". International Journal of Molecular Sciences. 13 (6): 7303–7327. doi:10.3390/ijms13067303. PMC 3397527. PMID 22837695.
- ^ a b c Ghiso J, Frangione B (December 2002). "Amyloidosis and Alzheimer's disease". Advanced Drug Delivery Reviews. 54 (12): 1539–1551. doi:10.1016/S0169-409X(02)00149-7. PMID 12453671.
- ^ a b Selkoe DJ (October 2001). "Clearing the brain's amyloid cobwebs". Neuron. 32 (2): 177–180. doi:10.1016/S0896-6273(01)00475-5. PMID 11683988. S2CID 17860343.
- ^ Hardy J, Duff K, Hardy KG, Perez-Tur J, Hutton M (September 1998). "Genetic dissection of Alzheimer's disease and related dementias: amyloid and its relationship to tau". Nature Neuroscience. 1 (5): 355–358. doi:10.1038/1565. PMID 10196523. S2CID 52807658.
- ^ Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, et al. (October 1999). "Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE". Science. 286 (5440): 735–741. doi:10.1126/science.286.5440.735. PMID 10531052. S2CID 42481897.
- ^ Vassar R (December 2002). "Beta-secretase (BACE) as a drug target for Alzheimer's disease". Advanced Drug Delivery Reviews. 54 (12): 1589–1602. doi:10.1016/S0169-409X(02)00157-6. PMID 12453676.
- ^ Zlokovic BV, Frangione B (2003). Transport-clearance hypothesis for Alzheimer's disease and potential therapeutic implications. Landes Bioscience. pp. 114–122.
- ^ Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K (June 1985). "Amyloid plaque core protein in Alzheimer disease and Down syndrome". Proceedings of the National Academy of Sciences of the United States of America. 82 (12): 4245–4249. Bibcode:1985PNAS...82.4245M. doi:10.1073/pnas.82.12.4245. PMC 397973. PMID 3159021.
- ^ Castaño EM, Prelli F, Soto C, Beavis R, Matsubara E, Shoji M, et al. (December 1996). "The length of amyloid-beta in hereditary cerebral hemorrhage with amyloidosis, Dutch type. Implications for the role of amyloid-beta 1-42 in Alzheimer's disease". The Journal of Biological Chemistry. 271 (50): 32185–32191. doi:10.1074/jbc.271.50.32185. PMID 8943274.
- ^ Roher AE, Lowenson JD, Clarke S, Woods AS, Cotter RJ, Gowing E, et al. (November 1993). "beta-Amyloid-(1-42) is a major component of cerebrovascular amyloid deposits: implications for the pathology of Alzheimer disease". Proceedings of the National Academy of Sciences of the United States of America. 90 (22): 10836–10840. Bibcode:1993PNAS...9010836R. doi:10.1073/pnas.90.22.10836. PMC 47873. PMID 8248178.
- ^ Lue LF, Kuo YM, Roher AE, Brachova L, Shen Y, Sue L, et al. (September 1999). "Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer's disease". The American Journal of Pathology. 155 (3): 853–862. doi:10.1016/S0002-9440(10)65184-X. PMC 1866907. PMID 10487842.
- ^ Schirinzi T, Di Lazzaro G, Sancesario GM, Colona VL, Scaricamazza E, Mercuri NB, et al. (December 2017). "Levels of amyloid-beta-42 and CSF pressure are directly related in patients with Alzheimer's disease". Journal of Neural Transmission. 124 (12): 1621–1625. doi:10.1007/s00702-017-1786-8. PMID 28866757. S2CID 22267507.
- ^ Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, et al. (April 2003). "Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis". Science. 300 (5618): 486–489. Bibcode:2003Sci...300..486K. doi:10.1126/science.1079469. hdl:2027.42/150615. PMID 12702875. S2CID 29614957.
- ^ Jin WS, Bu XL, Liu YH, Shen LL, Zhuang ZQ, Jiao SS, et al. (February 2017). "Plasma Amyloid-Beta Levels in Patients with Different Types of Cancer". Neurotoxicity Research. 31 (2): 283–288. doi:10.1007/s12640-016-9682-9. PMID 27913965. S2CID 3795042.
- ^ Lim S, Yoo BK, Kim HS, Gilmore HL, Lee Y, Lee HP, et al. (December 2014). "Amyloid-β precursor protein promotes cell proliferation and motility of advanced breast cancer". BMC Cancer. 14: 928. doi:10.1186/1471-2407-14-928. PMC 4295427. PMID 25491510.
- ^ Hartley SL, Handen BL, Devenny D, Mihaila I, Hardison R, Lao PJ, et al. (October 2017). "Cognitive decline and brain amyloid-β accumulation across 3 years in adults with Down syndrome". Neurobiology of Aging. 58: 68–76. doi:10.1016/j.neurobiolaging.2017.05.019. PMC 5581712. PMID 28715661.
- ^ Olsson F, Schmidt S, Althoff V, Munter LM, Jin S, Rosqvist S, et al. (January 2014). "Characterization of intermediate steps in amyloid beta (Aβ) production under near-native conditions". The Journal of Biological Chemistry. 289 (3): 1540–1550. doi:10.1074/jbc.M113.498246. PMC 3894335. PMID 24225948.
- ^ Hartmann T, Bieger SC, Brühl B, Tienari PJ, Ida N, Allsop D, et al. (September 1997). "Distinct sites of intracellular production for Alzheimer's disease A beta40/42 amyloid peptides". Nature Medicine. 3 (9): 1016–1020. doi:10.1038/nm0997-1016. PMID 9288729. S2CID 8390460.
- ^ Alzheimer's Association (March 2008). "2008 Alzheimer's disease facts and figures". Alzheimer's & Dementia. 4 (2): 110–133. doi:10.1016/j.jalz.2008.02.005. PMID 18631956. S2CID 43750218.
- ^ De Jonghe C, Esselens C, Kumar-Singh S, Craessaerts K, Serneels S, Checler F, et al. (August 2001). "Pathogenic APP mutations near the gamma-secretase cleavage site differentially affect Abeta secretion and APP C-terminal fragment stability". Human Molecular Genetics. 10 (16): 1665–1671. doi:10.1093/hmg/10.16.1665. PMID 11487570.
- ^ Chartier-Harlin MC, Crawford F, Houlden H, Warren A, Hughes D, Fidani L, et al. (October 1991). "Early-onset Alzheimer's disease caused by mutations at codon 717 of the beta-amyloid precursor protein gene". Nature. 353 (6347): 844–846. Bibcode:1991Natur.353..844C. doi:10.1038/353844a0. PMID 1944558. S2CID 4345311.
- ^ Lantos PL, Luthert PJ, Hanger D, Anderton BH, Mullan M, Rossor M (March 1992). "Familial Alzheimer's disease with the amyloid precursor protein position 717 mutation and sporadic Alzheimer's disease have the same cytoskeletal pathology". Neuroscience Letters. 137 (2): 221–224. doi:10.1016/0304-3940(92)90408-y. PMID 1584463. S2CID 25383047.
- ^ Lloyd GM, Trejo-Lopez JA, Xia Y, McFarland KN, Lincoln SJ, Ertekin-Taner N, et al. (March 2020). "Prominent amyloid plaque pathology and cerebral amyloid angiopathy in APP V717I (London) carrier - phenotypic variability in autosomal dominant Alzheimer's disease". Acta Neuropathologica Communications. 8 (1): 31. doi:10.1186/s40478-020-0891-3. PMC 7068954. PMID 32164763.
- ^ Glenner GG, Wong CW (August 1984). "Alzheimer's disease and Down's syndrome: sharing of a unique cerebrovascular amyloid fibril protein". Biochemical and Biophysical Research Communications. 122 (3): 1131–1135. doi:10.1016/0006-291X(84)91209-9. PMID 6236805.
- ^ Zhang S, Iwata K, Lachenmann MJ, Peng JW, Li S, Stimson ER, et al. (June 2000). "The Alzheimer's peptide a beta adopts a collapsed coil structure in water". Journal of Structural Biology. 130 (2–3): 130–141. doi:10.1006/jsbi.2000.4288. PMID 10940221.
- ^ Yang M, Teplow DB (December 2008). "Amyloid beta-protein monomer folding: free-energy surfaces reveal alloform-specific differences". Journal of Molecular Biology. 384 (2): 450–464. doi:10.1016/j.jmb.2008.09.039. PMC 2673916. PMID 18835397.
- ^ Sgourakis NG, Merced-Serrano M, Boutsidis C, Drineas P, Du Z, Wang C, et al. (January 2011). "Atomic-level characterization of the ensemble of the Aβ(1-42) monomer in water using unbiased molecular dynamics simulations and spectral algorithms". Journal of Molecular Biology. 405 (2): 570–583. doi:10.1016/j.jmb.2010.10.015. PMC 3060569. PMID 21056574.
- ^ Sgourakis NG, Yan Y, McCallum SA, Wang C, Garcia AE (May 2007). "The Alzheimer's peptides Abeta40 and 42 adopt distinct conformations in water: a combined MD / NMR study". Journal of Molecular Biology. 368 (5): 1448–1457. doi:10.1016/j.jmb.2007.02.093. PMC 1978067. PMID 17397862.
- ^ Ahmed M, Davis J, Aucoin D, Sato T, Ahuja S, Aimoto S, et al. (May 2010). "Structural conversion of neurotoxic amyloid-beta(1-42) oligomers to fibrils". Nature Structural & Molecular Biology. 17 (5): 561–567. doi:10.1038/nsmb.1799. PMC 2922021. PMID 20383142.
- ^ Yu L, Edalji R, Harlan JE, Holzman TF, Lopez AP, Labkovsky B, et al. (March 2009). "Structural characterization of a soluble amyloid beta-peptide oligomer". Biochemistry. 48 (9): 1870–1877. doi:10.1021/bi802046n. PMID 19216516.
- ^ Strodel B, Lee JW, Whittleston CS, Wales DJ (September 2010). "Transmembrane structures for Alzheimer's Aβ(1-42) oligomers". Journal of the American Chemical Society. 132 (38): 13300–13312. doi:10.1021/ja103725c. PMID 20822103.
- ^ Cummings J, Lee G, Mortsdorf T, Ritter A, Zhong K (September 2017). "Alzheimer's disease drug development pipeline: 2017". review. Alzheimer's & Dementia. 3 (3): 367–384. doi:10.1016/j.trci.2017.05.002. PMC 5651419. PMID 29067343.
- ^ Schilling S, Rahfeld JU, Lues I, Lemere CA (May 2018). "Passive Aβ Immunotherapy: Current Achievements and Future Perspectives". review. Molecules. 23 (5): 1068. doi:10.3390/molecules23051068. PMC 6099643. PMID 29751505.
- ^ Heurling K, Leuzy A, Zimmer ER, Lubberink M, Nordberg A (February 2016). "Imaging β-amyloid using [(18)F]flutemetamol positron emission tomography: from dosimetry to clinical diagnosis". European Journal of Nuclear Medicine and Molecular Imaging. 43 (2): 362–373. doi:10.1007/s00259-015-3208-1. PMID 26440450. S2CID 2695342.
- ^ Ito H, Shimada H, Shinotoh H, Takano H, Sasaki T, Nogami T, et al. (June 2014). "Quantitative Analysis of Amyloid Deposition in Alzheimer Disease Using PET and the Radiotracer ¹¹C-AZD2184". Journal of Nuclear Medicine. 55 (6): 932–938. doi:10.2967/jnumed.113.133793. PMID 24732152.
- ^ Schmidt SD, Nixon RA, Mathews PM (2012). "Tissue Processing Prior to Analysis of Alzheimer's Disease Associated Proteins and Metabolites, Including Aβ". Amyloid Proteins. Methods in Molecular Biology. Vol. 849. pp. 493–506. doi:10.1007/978-1-61779-551-0_33. ISBN 978-1-61779-550-3. PMID 22528111.
- ^ Schmidt SD, Mazzella MJ, Nixon RA, Mathews PM (2012). "Aβ Measurement by Enzyme-Linked Immunosorbent Assay". Amyloid Proteins. Methods in Molecular Biology. Vol. 849. pp. 507–27. doi:10.1007/978-1-61779-551-0_34. ISBN 978-1-61779-550-3. PMID 22528112.
- ^ Stine WB, Dahlgren KN, Krafft GA, LaDu MJ (March 2003). "In vitro characterization of conditions for amyloid-beta peptide oligomerization and fibrillogenesis". The Journal of Biological Chemistry. 278 (13): 11612–11622. doi:10.1074/jbc.M210207200. PMID 12499373.
- ^ Lasch P, Kneipp J (2008). Biomedical Vibrational Spectroscopy. Wiley. ISBN 978-0-470-22945-3.
- ^ Benseny-Cases N, Klementieva O, Cotte M, Ferrer I, Cladera J (December 2014). "Microspectroscopy (μFTIR) reveals co-localization of lipid oxidation and amyloid plaques in human Alzheimer disease brains". Analytical Chemistry. 86 (24): 12047–12054. doi:10.1021/ac502667b. PMID 25415602.
- ^ Gengler S, Gault VA, Harriott P, Hölscher C (June 2007). "Impairments of hippocampal synaptic plasticity induced by aggregated beta-amyloid (25-35) are dependent on stimulation-protocol and genetic background". Experimental Brain Research. 179 (4): 621–630. doi:10.1007/s00221-006-0819-6. PMID 17171334. S2CID 41040399.
- ^ Rekas A, Jankova L, Thorn DC, Cappai R, Carver JA (December 2007). "Monitoring the prevention of amyloid fibril formation by alpha-crystallin. Temperature dependence and the nature of the aggregating species". The FEBS Journal. 274 (24): 6290–6304. doi:10.1111/j.1742-4658.2007.06144.x. PMID 18005258. S2CID 85794556.
- ^ Sanghera N, Swann MJ, Ronan G, Pinheiro TJ (October 2009). "Insight into early events in the aggregation of the prion protein on lipid membranes". Biochimica et Biophysica Acta (BBA) - Biomembranes. 1788 (10): 2245–2251. doi:10.1016/j.bbamem.2009.08.005. PMID 19703409.
