Alkaloid: Difference between revisions
m Reverted edits by 84.73.185.168 to last version by BodhisattvaBot (HG) |
Schmutz MDPI (talk | contribs) |
||
| Line 52: | Line 52: | ||
* [[Natural products]] |
* [[Natural products]] |
||
* [[Secondary metabolite]] |
* [[Secondary metabolite]] |
||
* [[http://mdpi.com/journal/molecules/special_issues/alkaloids Special Issue: Alkaloids]] |
|||
{{IPA|}} |
{{IPA|}} |
||
Revision as of 07:57, 15 October 2008
This article is about the chemical compounds alkaloids. For the pharmaceutical company in the Republic of Macedonia see Alkaloid (company).
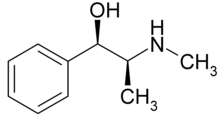
Alkaloids are naturally occurring chemical compounds containing basic nitrogen atoms.[1] The name derives from the word alkaline and was used to describe any nitrogen-containing base. Alkaloids are produced by a large variety of organisms, including bacteria, fungi, plants, and animals and are part of the group of natural products (also called secondary metabolites). Many alkaloids can be purified from crude extracts by acid-base extraction. Many alkaloids are toxic to other organisms. They often have pharmacological effects and are used as medications and recreational drugs. Examples are the local anesthetic and stimulant cocaine, the stimulant caffeine, nicotine, the analgesic morphine, or the antimalarial drug quinine. Some alkaloids have a bitter taste.
Alkaloid classifications
Alkaloids are usually classified by their common molecular precursors, based on the metabolic pathway used to construct the molecule. When not much was known about the biosynthesis of alkaloids, they were grouped under the names of known compounds, even some non-nitrogenous ones (since those molecules' structures appear in the finished product; the opium alkaloids are sometimes called "phenanthrenes", for example), or by the plants or animals they were isolated from. When more is learned about a certain alkaloid, the grouping is changed to reflect the new knowledge, usually taking the name of a biologically-important amine that stands out in the synthesis process.
- Pyridine group: piperine, coniine, trigonelline, arecoline, arecaidine, guvacine, cytisine, lobeline, nicotine, anabasine, sparteine, pelletierine.
- Pyrrolidine group: hygrine, cuscohygrine, nicotine
- Tropane group: atropine, cocaine, ecgonine, scopolamine, catuabine
- Quinoline group: quinine, quinidine, dihydroquinine, dihydroquinidine, strychnine, brucine, veratrine, cevadine
- Isoquinoline group: opium alkaloids (papaverine, narcotine, narceine), sanguinarine, hydrastine, berberine, emetine, berbamine, oxyacanthine
- Phenanthrene alkaloids: The opium alkaloids (morphine, codeine, thebaine)
- Phenethylamine group: mescaline, ephedrine, dopamine
- Indole group:
- Tryptamines: serotonin, DMT, 5-MeO-DMT, bufotenine, psilocybin
- Ergolines (the ergot alkaloids): ergine, ergotamine, lysergic acid, LSD
- Beta-carbolines: harmine, harmaline, tetrahydroharmine
- Yohimbans: reserpine, yohimbine
- Vinca alkaloids: vinblastine, vincristine
- Mitragyna speciosa alkaloids: mitragynine, 7-hydroxymitragynine
- Tabernanthe iboga alkaloids: ibogaine, voacangine, coronaridine, 18-methoxycoronaridine
- Strychnos nux-vomica alkaloids: strychnine, brucine
- Purine group:
- Terpenoid group:
- Aconite alkaloids: aconitine
- Steroid alkaloids (containing a steroid skeleton in a nitrogen containing structure):
- solanum (e.g. potato and tomato) alkaloids (solanidine, solanine, chaconine)
- veratrum alkaloids (veratramine, cyclopamine, cycloposine, jervine, muldamine)[2]
- newt alkaloids (samandarin)
- others conessine
- Quaternary ammonium compounds: muscarine, choline, neurine
- Miscellaneous: capsaicin, cynarin, phytolaccine, phytolaccotoxin
Physicochemical properties
Low-molecular weight alkaloids without hydrogen bond donors such as hydroxy groups are often liquid at room temperature, examples are nicotine, sparteine, coniine, and phenethylamine.
The basicity of alkaloids depends on the lone pairs of electrons on their nitrogen atoms. As organic bases, alkaloids form salts with mineral acids such as hydrochloric acid and sulfuric acid and organic acids such as tartaric acid or maleic acid. These salts are usually more water-soluble than their free base form.
See also
References
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (1995) "alkaloids". doi:10.1351/goldbook.A00220
- ^ http://www.ansci.cornell.edu/plants/toxicagents/steroid.html,
