African trypanosomiasis: Difference between revisions
m Dating maintenance tags: {{Fact}} |
No edit summary |
||
| Line 26: | Line 26: | ||
==Signs and symptoms== |
==Signs and symptoms== |
||
African trypanosomiasis symptoms occur in two stages. The first stage, known as the haemolymphatic phase, is characterized by |
African trypanosomiasis symptoms occur in two stages. The first stage, known as the haemolymphatic phase, is characterized by swelling of the genitilia.. Fever is intermittent, with attacks lasting from a day to a week, separated by intervals of a few days to a month or longer. Invasion of the circulatory and lymphatic systems by the parasites is associated with severe [[Lymphadenopathy|swelling]] of [[lymph nodes]], often to tremendous sizes. [[Winterbottom's sign]], the tell-tale swollen lymph nodes along the back of the neck, may appear. Occasionally, a red sore called a chancre will develop at the location of the tsetse fly bite. If left untreated, the disease overcomes the host's defenses and can cause more extensive damage, broadening symptoms to include [[anemia]], endocrine, [[cardiac]], and [[kidney disfunctions]]. The second, [[Neurological disorder|neurological phase]], begins when the parasite invades the [[central nervous system]] by passing through the [[blood–brain barrier]]. Disruption of the sleep cycle is a leading symptom of this stage and is the one that gave the disease the name 'sleeping sickness.' Infected individuals experience a disorganized and fragmented 24-hour rhythm of the sleep-wake cycle, resulting in daytime sleep episodes and nighttime periods of wakefulness.<ref>{{cite journal |author=GB Lundkvist, K Kristensson, M Bentivoglio |title=Why Trypanosomes Cause Sleeping Sickness |journal=Physiology |volume=19 |pages=198–206 |year=2004 |doi=10.1152/physiol.00006.2004}}</ref> Other neurological symptoms include [[confusion]], [[tremor]], general muscle weakness, [[hemiparesis]] and [[paralysis]] of a limb. [[Parkinsonism|Parkinson]]-like movements might arise due to non-specific movement disorders and speech disorders. Individuals may also exhibit psychiatric symptoms such as irritability, psychotic reactions, aggressive behaviour, or [[apathy]] which can sometimes dominate the clinical diagnosis.<ref>{{cite journal |author=Brun R, Blum J, Chappuis F, Burri C |title=Human African trypanosomiasis |journal=Lancet |volume=375 |pages=148–159 |year=2010 |doi=10.1016/S0140-6736(09)60829-1}}</ref> Without treatment, the disease is invariably fatal, with progressive mental deterioration leading to coma, systemic organ failure, and death. An untreated infection with ''[[Trypanosoma brucei rhodesiense|T.b. rhodesiense]]'' will cause death within months<ref>http://www.cdc.gov/parasites/sleepingsickness/gen_info/faqs-east.html</ref> whereas an untreated infection with ''[[Trypanosoma brucei gambiense|T.b. gambiense]]'' will cause death after several years.<ref>http://www.cdc.gov/parasites/sleepingsickness/gen_info/faqs-west.html</ref> Damage caused in the neurological phase is irreversible.<ref name="allafrica.com"/> |
||
[[Tryptophol]] is a chemical compound that induces sleep in humans. It |
[[Tryptophol]] is a chemical compound that induces sleep in humans. It |
||
Revision as of 14:11, 22 April 2013
| African trypanosomiasis | |
|---|---|
| Specialty | Infectious diseases |
Human African trypanosomiasis, sleeping sickness,[1] African lethargy,[1] or Congo trypanosomiasis[1] is a parasitic disease of people and animals, caused by protozoa of the species Trypanosoma brucei and transmitted by the tsetse fly.[2] There are two subspecies that infect humans, T.b. gambiense and T.b. rhodesiense, with the former accounting for over 95% of reported cases and the latter accounting for the remaining reported cases.[3] The disease is endemic in some regions of sub-Saharan Africa, covering areas in 36 countries containing more than 60 million people. A recent study estimates that the total African population at risk of contracting sleeping sickness is 69.3 million, with one third of this population being at a 'very high' to 'moderate' risk and the remaining two thirds at a 'low' to 'very low' risk.[4] The number of reported cases dropped below 10,000 in 2009, the first time in 50 years, and this downward trend was maintained in 2010. An estimated 30,000 people are currently infected.[3] Many cases are believed to go unreported. About 48,000 people died of sleeping sickness in 2008.[5] Four major epidemics have occurred in recent history: one from 1896 to 1906 primarily in Uganda and the Congo Basin, two epidemics in 1920 and 1970 in several African countries, and a recent 2008 epidemic in Uganda.[6]
Signs and symptoms
African trypanosomiasis symptoms occur in two stages. The first stage, known as the haemolymphatic phase, is characterized by swelling of the genitilia.. Fever is intermittent, with attacks lasting from a day to a week, separated by intervals of a few days to a month or longer. Invasion of the circulatory and lymphatic systems by the parasites is associated with severe swelling of lymph nodes, often to tremendous sizes. Winterbottom's sign, the tell-tale swollen lymph nodes along the back of the neck, may appear. Occasionally, a red sore called a chancre will develop at the location of the tsetse fly bite. If left untreated, the disease overcomes the host's defenses and can cause more extensive damage, broadening symptoms to include anemia, endocrine, cardiac, and kidney disfunctions. The second, neurological phase, begins when the parasite invades the central nervous system by passing through the blood–brain barrier. Disruption of the sleep cycle is a leading symptom of this stage and is the one that gave the disease the name 'sleeping sickness.' Infected individuals experience a disorganized and fragmented 24-hour rhythm of the sleep-wake cycle, resulting in daytime sleep episodes and nighttime periods of wakefulness.[7] Other neurological symptoms include confusion, tremor, general muscle weakness, hemiparesis and paralysis of a limb. Parkinson-like movements might arise due to non-specific movement disorders and speech disorders. Individuals may also exhibit psychiatric symptoms such as irritability, psychotic reactions, aggressive behaviour, or apathy which can sometimes dominate the clinical diagnosis.[8] Without treatment, the disease is invariably fatal, with progressive mental deterioration leading to coma, systemic organ failure, and death. An untreated infection with T.b. rhodesiense will cause death within months[9] whereas an untreated infection with T.b. gambiense will cause death after several years.[10] Damage caused in the neurological phase is irreversible.[6]
Tryptophol is a chemical compound that induces sleep in humans. It is produced by the trypanosomal parasite in sleeping sickness.[11]
Life cycle
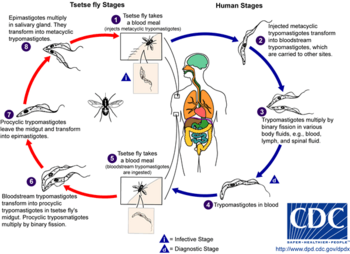
The tsetse fly (genus Glossina) is a large, brown, biting fly that serves as both a host and vector for the trypanosome parasites. While taking blood from a mammalian host, an infected tsetse fly injects metacyclic trypomastigotes into skin tissue. From the bite, parasites first enter the lymphatic system and then pass into the bloodstream. Inside the mammalian host, they transform into bloodstream trypomastigotes, and are carried to other sites throughout the body, reach other body fluids (e.g., lymph, spinal fluid), and continue to replicate by binary fission.
The entire life cycle of African trypanosomes is represented by extracellular stages. A tsetse fly becomes infected with bloodstream trypomastigotes when taking a blood meal on an infected mammalian host. In the fly's midgut, the parasites transform into procyclic trypomastigotes, multiply by binary fission, leave the midgut, and transform into epimastigotes. The epimastigotes reach the fly's salivary glands and continue multiplication by binary fission.
The entire life cycle of the fly takes about three weeks.
In addition to the bite of the tsetse fly, the disease can be transmitted by:
- Mother-to-child infection: the trypanosome can sometimes cross the placenta and infect the fetus.[12]
- Laboratories: accidental infections, for example, through the handling of blood of an infected person and organ transplantation, although this is uncommon.
- Blood transfusion
- Sexual contact (This may be possible)[13]
Diagnosis

The gold standard for diagnosis is identification of trypanosomes in a patient sample by microscopic examination. Patient samples that can be used for diagnosis include chancre fluid, lymph node aspirates, blood, bone marrow, and, during the neurological stage, cerebrospinal fluid. Detection of trypanosome-specific antibodies can be used for diagnosis, but the sensitivity and specificity of these methods are too variable to be used alone for clinical diagnosis. Further, seroconversion occurs after the onset of clinical symptoms during a T. b. rhodesiense infection, so is of limited diagnostic use.[citation needed]
Trypanosomes can be detected from patient samples using two different preparations. A wet preparation can be used to look for the motile trypanosomes. Alternatively, a fixed (dried) smear can be stained with Giemsa (or Field) and examined. Often, the parasite is in relatively low abundance in the sample, so techniques to concentrate the parasites can be used prior to microscopic examination. For blood samples, these include centrifugation followed by examination of the buffy coat; mini anion-exchange/centrifugation; and the quantitative buffy coat (QBC) technique. For other samples, such as spinal fluid, concentration techniques include centrifugation followed by examination of the sediment.[citation needed]
Three serological tests are also available for detection of the parasite: the micro-CATT, wb-CATT, and wb-LATEX. The first uses dried blood, while the other two use whole blood samples. A 2002 study found the wb-CATT to be the most efficient for diagnosis, while the wb-LATEX is a better exam for situations where greater sensitivity is required.[14]
Prevention
Currently there are few medically related prevention options for African Trypanosomiasis (i.e. no vaccine exists for immunity). Although the risk of infection from a tsetse fly bite is minor (estimated at less than 0.1%), the use of insect repellants, wearing long-sleeved clothing, avoiding tsetse-dense areas, implementing bush clearance methods and wild game culling are the best options to avoid infection available for local residents of the disease [15] At the 25th ISCTRC (International Scientific Council for Trypanosomiasis Research and Control) in Mombasa, Kenya, in October, 1999, the idea of an African-wide initiative to control tsetse and trypanosomiasis populations was discussed. During the 36th summit of the African Union in Lome, Togo, in July 2000, a resolution was passed to form the Pan African Tsetse and Trypanosomiasis Eradication Campaign (PATTEC). The campaign works to eradicate the tsetse vector population levels and subsequently the protozoan disease, by use of insecticide-impregnated targets, fly traps, insecticide-treated cattle, ultra-low dose aerial/ground spraying (SAT) of tsetse resting sites and the sterile insect technique(SIT).[16] The use of SIT in Zanzibar proved effective in eliminating the entire population of tsetse flies but was expensive and is relatively impractical to use in many of the endemic countries afflicted with African trypanosomiasis.[15]
Regular active surveillance, involving detection and prompt treatment of new infections, and tsetse fly control is the backbone of the strategy used to control sleeping sickness. Systematic screening of at-risk communities is the best approach, because case-by-case screening is not practical in endemic regions. Systematic screening may be in the form of mobile clinics or fixed screening centres where teams travel daily to areas of high infection rates. Such screening efforts are important because early symptoms are not evident or serious enough to warrant patients with gambiense disease to seek medical attention, particularly in very remote areas. Also, diagnosis of the disease is difficult and health workers may not associate such general symptoms with trypanosomiasis. Systematic screening allows early-stage disease to be detected and treated before the disease progresses, and removes the potential human reservoir.[17] A single case of sexual transmission of West African sleeping sickness has been reported,[13]
Treatment
First line, first stage
The current standard treatment for first-stage (haemolymphatic) disease is intravenous or intramuscular pentamidine (for T.b. gambiense), or intravenous suramin (for T.b. rhodesiense).
The drug eflornithine — previously used only as an alternative treatment for sleeping sickness due to its labour-intensive administration — was found to be safe and effective as a first-line treatment for the disease in 2008, according to the Science and Development Network's sub-Saharan Africa news updates. [1]. Researchers tracked over 1,000 adults and children at a centre in Ibba, Southern Sudan—the first use of eflornithine on a large scale— and it was highly effective in treating the issue.[citation needed]
According to a treatment study of Trypanosoma gambiense-caused human African trypanosomiasis, use of eflornithine (DMFO) resulted in fewer adverse events than treatment with melarsoprol.[18]
First line, second stage
The current standard treatment for second-stage (neurological phase) disease is:
- Intravenous melarsoprol 2.2 mg/kg daily for 12 consecutive days[19]
Alternative first line therapies include:
- Intravenous melarsoprol 0.6 mg/kg on day 1, 1.2 mg/kg IV melarsoprol on day 2, and 1.2 mg/kg/day IV melarsoprol combined with oral 7.5 mg/kg nifurtimox twice a day on days 3 to 10[20] or
- Intravenous eflornithine 50 mg/kg every six hours for 14 days[21]
Combination therapy with eflornithine and nifurtimox is safer and easier than treatment with eflornithine alone, and appears to be equally or more effective. It has been recommended as first-line treatment for second-stage T. b. gambiensis disease.[22]
Resistant disease
In areas with melarsoprol resistance or in patients who have relapsed after melarsoprol monotherapy, the treatment should be melarsoprol and nifurtimox, or eflornithine.
Outdated protocols
The following traditional regimens should no longer be used:
- Old "standard" 26-day melarsoprol therapy: Intravenous melarsoprol therapy (three series of 3.6 mg/kg/day intravenously for 3 days, with 7-day breaks between the series) (this regimen is less convenient and patients are less likely to complete therapy)[23]
- Incremental melarsoprol therapy: 10-day incremental-dose melarsoprol therapy (0.6 mg/kg iv on day 1, 1.2 mg/kg IV on day 2, and 1.8 mg/kg IV on days 3–10) (previously thought to reduce the risk of treatment-induced encephalopathy, but now known to be associated with an increased risk of relapse and a higher incidence of encephalopathy)[20][23]
Epidemiology

There are two subspecies of the parasite that are responsible for initiating the disease in humans. Trypanosoma brucei gambiense causes the diseases in west and central Africa whereas, Trypanosoma brucei rhodesiense has a limited geographical range and is responsible for causing the disease in east and southern Africa. In addition, a third subspecies of the parasite known as Trypanosoma brucei brucei is responsible for affecting animals but not humans. Humans are the main reservoir for T. b. gambiense but this species can also be found in pigs and other animals. Wild game animals and cattle are the main reservoir of T. b. rhodesiense. These parasites primarily infect individuals in sub-Saharan Africa because that is where the vector (tsetse fly) is located. The two human forms of the disease also vary greatly in intensity. T. b. gambiense causes a chronic condition that can remain in a passive phase for months or years before symptoms emerge and the infection can last about 3 years before death occurs. T. b. rhodesiense is the acute form of the disease and death can occur within months since the symptoms emerge within weeks and it is more virulent and faster developing than T. b. gambiense. Furthermore, trypanosomes are surrounded by a coat that is composed of variant surface glycoproteins (VSG). These proteins act to protect the parasite from any lytic factors that are present in human plasma. The host’s immune system recognizes the glycoproteins present on the coat of the parasite leading to the production of different antibodies (IgM and IgG). These antibodies will then act to destroy the parasites that circulate around the blood. However, from the several parasites present in the plasma, a small number of them will experience changes in their surface coats resulting in the formation of new VSGs. Thus, the antibodies produced by the immune system will no longer recognize the parasite leading to proliferation until new antibodies are created to combat the novel VSGs. Eventually the immune system will no longer be able to fight off the parasite due to the constant changes in VSGs and infection will arise.[25]
According to recent estimates, the disability-adjusted life-years (9 to 10 years) lost due to sleeping sickness are 2.0 million.[26] Recent estimates indicate over 60 million people living in some 250 locations are at risk of contracting the disease, and under 10,000 new cases were reported in 2009 according to WHO figures, which represents a huge decrease from the estimated 300,000 new cases in 1998.[27] The disease has been recorded as occurring in 36 countries, all in sub-Saharan Africa. It is endemic in southeast Uganda and western Kenya, and killed more than 48,000 Africans in 2008.[6]
Horse-flies (Tabanidae) and stable flies (Muscidae) possibly play a role in transmission of nagana (the animal form of sleeping sickness) and the human disease form.[28]
History
The condition has been present in Africa since at least the 14th century, and probably for thousands of years before then. Because of a lack of travel between indigenous people, sleeping sickness in humans had been limited to isolated pockets. This changed once Arab slave traders entered central Africa from the east, following the Congo River, bringing parasites along. Gambian sleeping sickness travelled up the Congo River, then further eastwards. In 1901, a devastating epidemic erupted in Uganda, killing more than 250,000 people,[29] including about two-thirds of the population in the affected lakeshore areas. According to The Cambridge History of Africa, "It has been estimated that up to half the people died of sleeping-sickness and smallpox in the lands on either bank of the lower river Congo."[30]

The causative agent and vector were identified in 1903 by David Bruce, and the differentiation between the subspecies of the protozoa made in 1910. The first effective treatment, atoxyl, an arsenic-based drug developed by Paul Ehrlich and Kiyoshi Shiga, was introduced in 1910, but blindness was a serious side effect. Numerous drugs designed to treat the disease have been introduced since then.[citation needed]
Suramin was introduced in 1920 to treat the first stage of the disease. By 1922, Suramin was generally combined with tryparsamide (another pentavalent organoarsenic drug) in the treatment of the second stage of the gambiense form. It was used during the grand epidemic in West and Central Africa in millions of people and was the mainstay of therapy until 1969.[citation needed]
Pentamidine, a highly effective drug for the first stage of the disease, has been used since 1939. During the 1950s, it was widely used as a prophylactic agent in western Africa, leading to a sharp decline in infection rates. At the time, eradication of the disease was thought to be at hand.[citation needed]
The organoarsenical melarsoprol (Arsobal) developed in the 1940s is effective for patients with second-stage sleeping sickness. However, 3-10% of those injected have reactive encephalopathy (convulsions, progressive coma, or psychotic reactions), and 10-70% of such cases result in death; it can cause brain damage in those who survive the encephalopathy. However, due to its effectiveness, melarsoprol is still used today. Resistance to melarsoprol is increasing, and combination therapy with nifurtimox is currently under research.[citation needed]
Eflornithine (difluoromethylornithine or DFMO), the most modern treatment, was developed in the 1970s by Albert Sjoerdsmanot and underwent clinical trials in the 1980s. The drug was approved by the United States Food and Drug Administration in 1990, but Aventis, the company responsible for its manufacture, halted production in 1999. In 2001, however, Aventis, in association with Médecins Sans Frontières and the World Health Organization, signed a long-term agreement to manufacture and donate the drug.[citation needed]
Research
The genome of the parasite has been sequenced and several proteins have been identified as potential targets for drug treatment. Analysis of the genome also revealed the reason why generating a vaccine for this disease has been so difficult. T. brucei has over 800 genes that make proteins the parasite "mixes and matches" to evade immune system detection.[31]
Recent findings indicate the parasite is unable to survive in the bloodstream without its flagellum. This insight gives researchers a new angle with which to attack the parasite.[32]
A new treatment based on a truncated version of the apolipoprotein L-1 of high density lipoprotein and a single-domain antibody has recently been found to work in mice, but has not been tested in humans.[33]
The cover story of the August 25, 2006, issue of the journal Cell describes an advance in understanding how Trypanosomes escape the immune system. Dr. Lee Soo Hee and colleagues, working at Johns Hopkins investigated the pathway by which trypanosomes make myristate, a 14-carbon length fatty acid. Myristate is a component of the variant surface glycoprotein (VSG), the molecule that makes up the trypanosome's outer layer. This outer surface coat of VSG is vital to the trypanosome's ability to avoid destruction by the host's immune system. Dr. Lee and colleagues discovered trypanosomes use a novel fatty acid synthesis pathway involving fatty acid elongases to make myristate and other fatty acids.
An international research team working in the Democratic Republic of the Congo, Southern Sudan, and Angola involving Immtech International and University of North Carolina at Chapel Hill have completed a Phase IIb clinical trial and began a Phase III trial in 2005 testing the efficacy of the first oral treatment for sleeping sickness, pafuramidine (DB289).[34][35] Trypanosomiasis vaccines are undergoing research.
Two independent variants of the APOL1 gene found in African haplotypes carrying signatures of natural selection have been shown to confer protection against the acute version of sleeping sickness caused by T. b. rhodesiense, while at the same time increasing risk of kidney disease when inherited from both parents.[36]
Synthetic and computer-based approaches are used for the development of newer antitrypanosomal analogues with improved efficacy and oral bioavailability.[37]
The Dempster-Shafer theory for detecting African trypanosomiasis displays the result of detection process.[38]
A genetic modification of the bacteria Sodalis glossinidius (present inside the tsetse fly) is also being examined as a possible weapon against the disease.[39]
See also
- Human trypanosomiasis
- Drugs for Neglected Diseases Initiative
- Sleep disorder
- Chagas disease (American trypanosomiasis), another human tropical disease caused by trypanosomes
- Nagana (animal African trypanosomiasis)
References
- ^ a b c Robinson, Victor, ed. (1939). "African Lethargy, Sleeping Sickness, or Congo trypanosomiasis; Trypanosoma gambiense". The Modern Home Physician, A New Encyclopedia of Medical Knowledge. WM. H. Wise & Company (New York)., pp. 20–21.
- ^ MedlinePlus Encyclopedia: Sleeping sickness
- ^ a b WHO Media centre (2012). "Fact sheet N°259: Trypanosomiasis, Human African (sleeping sickness)".
{{cite journal}}: Cite journal requires|journal=(help) - ^ Simarro PP, Cecchi G, Franco JR, Paone M, Diarra A, Ruiz-Postigo JA, Fèvre EM, Mattioli RC, Jannin JG (2012). "Estimating and Mapping the Population at Risk of Sleeping Sickness". PLoS Negl Trop Dis. 6 (10): e1859. doi:10.1371/journal.pntd.0001859.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ "New treatments raise hope of cutting sleeping sickness deaths". The Guardian. May 15, 2009.
- ^ a b c "Uganda: Sleeping Sickness Reaching Alarming Levels," New Vision, May 11, 2008.
- ^ GB Lundkvist, K Kristensson, M Bentivoglio (2004). "Why Trypanosomes Cause Sleeping Sickness". Physiology. 19: 198–206. doi:10.1152/physiol.00006.2004.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Brun R, Blum J, Chappuis F, Burri C (2010). "Human African trypanosomiasis". Lancet. 375: 148–159. doi:10.1016/S0140-6736(09)60829-1.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ http://www.cdc.gov/parasites/sleepingsickness/gen_info/faqs-east.html
- ^ http://www.cdc.gov/parasites/sleepingsickness/gen_info/faqs-west.html
- ^ Cornford, E M; Bocash, W D; Braun, L D; Crane, P D; Oldendorf, W H; MacInnis, A J (1979). "Rapid distribution of tryptophol (3-indole ethanol) to the brain and other tissues". Journal of Clinical Investigation. 63 (6): 1241–8. doi:10.1172/JCI109419. PMC 372073. PMID 447842.
- ^ Olowe SA (1975). "A case of congenital trypanosomiasis in Lagos". Trans. R. Soc. Trop. Med. Hyg. 69 (1): 57–9. doi:10.1016/0035-9203(75)90011-5. PMID 1170654.
- ^ a b Rocha G, Martins A, Gama G, Brandão F, Atouguia J (2004). "Possible cases of sexual and congenital transmission of sleeping sickness". Lancet. 363 (9404): 247. doi:10.1016/S0140-6736(03)15345-7. PMID 14738812.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Truc P; Lejon V; Magnus E; et al. (2002). "Evaluation of the micro-CATT, CATT/Trypanosoma brucei gambiense, and LATEX/T b gambiense methods for serodiagnosis and surveillance of human African trypanosomiasis in West and Central Africa". Bull. World Health Organ. 80 (11): 882–6. PMC 2567684. PMID 12481210. Retrieved 2009-03-16.
{{cite journal}}: Unknown parameter|author-separator=ignored (help) - ^ a b Brun R, Blum J, Chappuis F, Burri C (2010). "Human African trypanosomiasis". Lancet. 375: 154–155.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Schofield C, Kabayo J (2008). "Trypanosomiasis vector control in Africa and Latin America". Parasites and Vectors. 1: 4–5.
- ^ "Strategic Direction for African Trypanosomiasis Research". Special Programme for Research and Training in Tropical Diseases. Archived from the original on 22 March 2006. Retrieved 2006-03-01.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Chappuis F, Udayraj N, Stietenroth K, Meussen A, Bovier PA (2005). "Eflornithine is safer than melarsoprol for the treatment of second-stage Trypanosoma brucei gambiense human African trypanosomiasis". Clin. Infect. Dis. 41 (5): 748–51. doi:10.1086/432576. PMID 16080099.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Burri, C; Nkunku, S; Merolle, A; Smith, T; Blum, J; Brun, R (2000). "Efficacy of new, concise schedule for melarsoprol in treatment of sleeping sickness caused by Trypanosoma brucei gambiense: a randomised trial". Lancet. 355 (9213): 1419–25. doi:10.1016/S0140-6736(00)02141-3. PMID 10791526.
{{cite journal}}: Unknown parameter|unused_data=ignored (help) - ^ a b Bisser S; N'Siesi FX; Lejon V; et al. (2007). "Equivalence trial of melarsoprol and nifurtimox monotherapy and combination therapy for the treatment of second-stage Trypanosoma brucei gambiense sleeping sickness". J. Infect. Dis. 195 (3): 322–9. doi:10.1086/510534. PMID 17205469.
{{cite journal}}: Unknown parameter|author-separator=ignored (help) - ^ van Nieuwenhove S; Schechter PJ; Declercq J; et al. (1985). "Treatment of gambiense sleeping sickness in the Sudan with oral DFMO (DL-alfa-difluoromethyl ornithine) an inhibitor of ornithine decarboxylase: first field trial". Trans R Soc Trop Med Hyg. 79 (5): 692–8. doi:10.1016/0035-9203(85)90195-6. PMID 3938090.
{{cite journal}}: Unknown parameter|author-separator=ignored (help) - ^ Priotto G; Kasparian S; Mutombo W; et al. (2009). "Nifurtimox-eflornithine combination therapy for second-stage African Trypanosoma brucei gambiense trypanosomiasis: a multicentre, randomised, phase III, non-inferiority trial". Lancet. 374 (9683): 56–64. doi:10.1016/S0140-6736(09)61117-X. PMID 19559476.
{{cite journal}}: Unknown parameter|author-separator=ignored (help); Unknown parameter|month=ignored (help) - ^ a b Pepin J, Mpia B (2006). "Randomized controlled trial of three regimens of melarsoprol in the treatment of Trypanosoma brucei gambiense trypanosomiasis". Trans R Soc Trop Med Hyg. 100 (5): 437–41. doi:10.1016/j.trstmh.2005.03.017. PMID 16483622.
- ^ WHO mortality and health data and statistics, accessed Feb 10, 2009.
- ^ Brun R, Blum J, Chappuis F, Burri C (2010). "Human African trypanosomiasis". Lancet. 375: 147–59.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ World Health Organization (Geneva) (2000). "World Health Report 2000: Health Systems Improving Performance".
{{cite journal}}: Cite journal requires|journal=(help) - ^ WHO Expert Committee on Control and Surveillance of African trypanosomiasis (Geneva) (1998). "WHO Technical Report Series,No.881".
{{cite journal}}: Cite journal requires|journal=(help) - ^ Cherenet T, Sani RA, Panandam JM, Nadzr S, Speybroeck N, van den Bossche P (2004). "Seasonal prevalence of bovine trypanosomosis in a tsetse-infested zone and a tsetse-free zone of the Amhara Region, north-west Ethiopia". The Onderstepoort journal of veterinary research. 71 (4): 307–312. PMID 15732457.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Fèvre EM, Coleman PG, Welburn SC, Maudlin I (2004). "Reanalyzing the 1900–1920 sleeping sickness epidemic in Uganda". Emerging Infect. Dis. 10 (4): 567–73. doi:10.3201/eid1004.020626. PMID 15200843.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Fage, John D. (5 September 1985). The Cambridge History of Africa: From the earliest times to c. 500 BC. Cambridge University Press. p. 748. ISBN 978-0-521-22803-9.
- ^ Berriman M; Ghedin E; Hertz-Fowler C; et al. (2005). "The genome of the African trypanosome Trypanosoma brucei". Science. 309 (5733): 416–22. Bibcode:2005Sci...309..416B. doi:10.1126/science.1112642. PMID 16020726.
{{cite journal}}: Unknown parameter|author-separator=ignored (help) - ^ "African Sleeping Sickness Breakthrough". Archived from the original on 13 May 2006. Retrieved April 7, 2006.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ New Scientist, 25 Aug. 2007, pp. 35-7
- ^ Williamson, David (August 25, 2005). "Compound might defeat African sleeping sickness, clinical trial beginning this month". University of North Carolina.
- ^ Staff (September 15, 2005). "Clinical Trials Update". Genetic Engineering News. p. 5.
- ^ Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, Bowden DW, Langefeld CD, Oleksyk TK, Uscinski Knob AL, Bernhardy AJ, Hicks PJ, Nelson GW, Vanhollebeke B, Winkler CA, Kopp JB, Pays E, Pollak MR. (2010). "Association of Trypanolytic ApoL1 Variants with Kidney Disease in African-Americans". Science. 329 (5993): 841–5. doi:10.1126/science.1193032. PMC 2980843. PMID 20647424.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Paliwal SK, Verma AN, Paliwal S (2011). "Neglected Disease – African Sleeping Sickness: Recent Synthetic and Modeling Advances". Sci. Pharm. 79 (3): 329–428. doi:10.3797/scipharm.1012-08. PMC 316337. PMID 21886894.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Maseleno A, Hasan M (2012). "African Trypanosomiasis Detection using Dempster-Shafer Theory". Journal of Emerging Trends in Computing and Information Sciences. 3 (4): 480–7. arXiv:1205.0831.
- ^ Bacterium transforms into weapon against sleeping sickness
External links
- Sleeping sickness information page at Médecins Sans Frontières
- Drugs for Neglected Diseases Initiative
- Medecins Sans Frontieres' Eflornithine press release, 2001
- Links to pictures of Sleeping Sickness (Hardin MD/ University of Iowa)
- The Sandler Center for Basic Research in Parasitic Diseases, University of California, San Francisco.
- Kids For World Health
- Eflornithine 'safe as first-line sleeping sickness treatment'
- Science without Frontiers
- Amaro RE, Swift RV, McCammon JA (2007). Keiser, Jennifer (ed.). "Functional and structural insights revealed by molecular dynamics simulations of an essential RNA editing ligase in Trypanosoma brucei". PLoS Negl Trop Dis. 1 (2): e68. doi:10.1371/journal.pntd.0000068. PMC 2100368. PMID 18060084.
Flash Video doi:10.4016/5585.01
{{cite journal}}: External link in|quote= - A Naturalist on Lake Victoria, with an Account of Sleeping Sickness and the Tse-tse Fly; 1920. T.F. Unwin Ltd, London; Biodiversity Archive
- Collection of articles from PLoS Neglected Tropical Diseases on sleeping sickness
