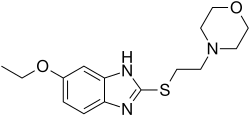
Fabomotizole
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Afobazole |
| Other names | Obenoxazine |
| Routes of administration | Oral |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 43.64%, pronounced first-pass effect |
| Metabolism | extensive hepatic |
| Onset of action | 0.85±0.13 hours |
| Elimination half-life | 0.82±0.54 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C15H21N3O2S |
| Molar mass | 307.41 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |

Fabomotizole (INN;[1] brand name Afobazole) is an anxiolytic drug launched in Russia in the early 2000s. It produces anxiolytic and neuroprotective effects without any sedative or muscle relaxant actions.[citation needed] Its mechanism of action remains poorly defined however, with GABAergic, NGF- and BDNF-release-promoting, MT1 receptor agonism, MT3 receptor antagonism, and sigma agonism suggested as potential mechanisms. Fabomotizole was shown to inhibit MAO-A reversibly and there might be also some involvement with serotonin receptors.[2][3][4][5][6] Clinical trials have shown fabomotizole to be well tolerated and reasonably effective for the treatment of anxiety.[7]
Experiments in mice have shown antimutagenic and antiteratogenic properties.[8]
Experiments in rats have shown beneficial effect in the model of ischemic stroke.[9]
Fabomotizole has found little clinical use outside Russia and has not been evaluated by the FDA.
See also
[edit]References
[edit]- ^ "International Nonproprietary Names for Pharmaceutical Substances (INN)" (PDF). WHO Drug Information. 26 (1): 63. 2012. Retrieved 21 March 2015.
- ^ Neznamov GG, Siuniakov SA, Chumakov DV, Bochkarev VK, Seredenin SB (2001). "[Clinical study of the selective anxiolytic agent afobazol]". Eksperimental'naia i Klinicheskaia Farmakologiia. 64 (2): 15–19. PMID 11548440.
- ^ Silkina IV, Gan'shina TC, Seredin SB, Mirzoian RS (2005). "[Gabaergic mechanism of cerebrovascular and neuroprotective effects of afobazole and picamilon]". Eksperimental'naia i Klinicheskaia Farmakologiia. 68 (1): 20–24. PMID 15786959.
- ^ Seredin SB, Melkumian DS, Val'dman EA, Iarkova MA, Seredina TC, Voronin MV, Lapitskaia AS (2006). "[Effects of afobazole on the BDNF content in brain structures of inbred mice with different phenotypes of emotional stress reaction]". Eksperimental'naia i Klinicheskaia Farmakologiia. 69 (3): 3–6. PMID 16878488.
- ^ Antipova TA, Sapozhnikova DS, Bakhtina LI, Seredenin SB (2009). "[Selective anxiolytic afobazole increases the content of BDNF and NGF in cultured hippocampal HT-22 line neurons]". Eksperimental'naia i Klinicheskaia Farmakologiia. 72 (1): 12–14. PMID 19334503.
- ^ Seredenin SB, Antipova TA, Voronin MV, Kurchashova SY, Kuimov AN (July 2009). "Interaction of afobazole with sigma1-receptors". Bulletin of Experimental Biology and Medicine. 148 (1): 42–44. doi:10.1007/s10517-009-0624-x. PMID 19902093. S2CID 37411324.
- ^ Medvedev VE, Trosnova AP, Dobrovol'skiĭ AV (2007). "[Psychopharmacotherapy of anxiety disorders in patients with cardio-vascular diseases: the use of aphobazole]". Zhurnal Nevrologii I Psikhiatrii Imeni S.S. Korsakova. 107 (7): 25–29. PMID 18379478.
- ^ Durnev AD, Zhanataev AK, Shreder OV, Seredenin SB (Jan–Feb 2009). "[Antimutagenic and antiteratogenic properties of afobazole]". Eksperimental'naia i Klinicheskaia Farmakologiia. 72 (1): 46–51. PMID 19334511.
- ^ Katnik C, Garcia A, Behensky AA, Yasny IE, Shuster AM, Seredenin SB, Petrov AV, Seifu S, McAleer J, Willing A, Cuevas J (February 2014). "Treatment with afobazole at delayed time points following ischemic stroke improves long-term functional and histological outcomes". Neurobiol Dis. 62: 354–364. doi:10.1016/j.nbd.2013.10.011. PMID 24141021.
