Aedes aegypti
| Yellow Fever Mosquito | |
|---|---|

| |
| Adult | |

| |
| Larva | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Diptera |
| Family: | Culicidae |
| Genus: | Aedes |
| Subgenus: | Stegomyia |
| Species: | A. aegypti
|
| Binomial name | |
| Aedes aegypti | |
| Subspecies[2][3] | |
| |
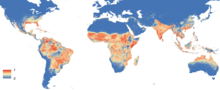
| |
| Global Aedes aegypti predicted distribution in 2015, (blue=absent, red=present) | |
| Synonyms[1] | |
Aedes aegypti (UK pronunciation: /ˈiːdiːz/; US pronunciation: /ˈeɪdz/ or /ˈeɪdiːz/ from Greek αηδής: "hateful" and /eɪˈdʒɪpti/ from Latin, meaning "of Egypt"), the yellow fever mosquito, is a mosquito that can spread dengue fever, chikungunya, Zika fever, Mayaro and yellow fever viruses, and other disease agents. The mosquito can be recognized by black and white markings on its legs and a marking in the form of a lyre on the upper surface of its thorax. This mosquito originated in Africa, but is now found in tropical, subtropical and temperate regions throughout the world.
Biology
[edit]
Aedes aegypti is a 4-to-7-millimetre-long (5⁄32 to 35⁄128 in), dark mosquito which can be recognized by white markings on its legs and a marking in the form of a lyre on the upper surface of its thorax. Females are larger than males. Microscopically females possess small palps tipped with silver or white scales, and their antennae have sparse short hairs, whereas those of males are feathery. Aedes aegypti can be confused with Aedes albopictus without a magnifying glass: the latter have a white stripe on the top of the mid thorax.[4]
Males live off fruit[5] and only the female bites for blood, which she needs to mature her eggs. To find a host, she is attracted to chemical compounds emitted by mammals, including ammonia,[6] carbon dioxide,[7] lactic acid, and octenol.[8] Scientists at The United States Department of Agriculture (USDA) Agricultural Research Service studied the specific chemical structure of octenol to better understand why this chemical attracts the mosquito to its host and found the mosquito has a preference for "right-handed" (dextrorotatory) octenol molecules.[9] The preference for biting humans is dependent on expression of the odorant receptor AaegOr4.[10] The white eggs are laid separately into water and not together, unlike most other mosquitoes, and soon turn black. The larvae feed on bacteria, growing over a period of weeks until they reach the pupa stage.[5]
The lifespan of an adult Ae. aegypti is two to four weeks depending on conditions,[11] but the eggs can be viable for over a year in a dry state, which allows the mosquito to re-emerge after a cold winter or dry spell.[12]
Hosts
[edit]Mammalian hosts include domesticated horses, and feral and wild horses and equids more generally.[13] As of 2009 birds were found to be the best food supply for Ae. aegypti among all taxa.[14]
Distribution
[edit]
Aedes aegypti originated in Africa and was spread to the New World through the slave trade,[15] but is now found in tropical, subtropical and temperate regions[16] throughout the world.[17] Ae. aegypti's distribution has increased in the past two to three decades worldwide, and it is considered to be among the most widespread mosquito species.[18]
In 2016, Zika virus-capable mosquito populations have been found adapting for persistence in warm temperate climates. Such a population has been identified to exist in parts of Washington, DC, and genetic evidence suggests they survived at least the last four winters in the region. One of the study researchers noted, "...some mosquito species are finding ways to survive in normally restrictive environments by taking advantage of underground refugia".[19] As the world's climate becomes warmer, the range of Aedes aegypti and a hardier species originating in Asia, the tiger mosquito Aedes albopictus, which can expand its range to relatively cooler climates, will inexorably spread north and south. Sadie Ryan of the University of Florida was the lead author in a 2019 study that estimated the vulnerability of naïve populations in geographic regions that currently do not harbor vectors i.e., for Zika in the Old World. Ryan's co-author, Georgetown University's Colin Carlson remarked,"Plain and simple, climate change is going to kill a lot of people."[20] As of 2020, the Northern Territory Government Australia and the Darwin City Council have recommended tropical cities initiate rectification programs to rid their cities of potential mosquito breeding stormwater sumps.[21] A 2019 study found that accelerating urbanization and human movement would also contribute to the spread of Aedes mosquitoes.[22]
In continental Europe, Aedes aegypti is not established but it has been found in localities close to Europe such as the Asian part of Turkey.[23] However, a single adult female specimen was found in Marseille (Southern France) in 2018. On the basis of a genetic study and an analysis of the movements of commercial ships, the origin of the specimen could be traced as coming from Cameroon, in Central Africa.[23]
Genomics
[edit]In 2007, the genome of Aedes aegypti was published, after it had been sequenced and analyzed by a consortium including scientists at The Institute for Genomic Research (now part of the J. Craig Venter Institute), the European Bioinformatics Institute, the Broad Institute, and the University of Notre Dame. The effort in sequencing its DNA was intended to provide new avenues for research into insecticides and possible genetic modification to prevent the spread of virus. This was the second mosquito species to have its genome sequenced in full (the first was Anopheles gambiae). The published data included the 1.38 billion base pairs containing the insect's estimated 15,419 protein-encoding genes. The sequence indicates the species diverged from Drosophila melanogaster (the common fruit fly) about 250 million years ago, and Anopheles gambiae and this species diverged about 150 million years ago.[24][25] Matthews et al., 2018 finds A. aegypti to carry a large and diverse number of transposable elements. Their analysis suggests this is common to all mosquitoes.[26]
Vector of disease
[edit]Aedes aegypti is a vector for transmitting numerous pathogens. According to the Walter Reed Biosystematics Units as of 2022,[27] it is associated with the following 54 viruses and 2 species of Plasmodium:
Aino virus (AINOV), African horse sickness virus (AHSV), Bozo virus (BOZOV), Bussuquara virus (BSQV), Bunyamwera virus (BUNV), Catu virus (CATUV), Chikungunya virus (CHIKV), Chandipura vesiculovirus (CHPV), Cypovirus (unnamed), Cache Valley virus (CVV), Dengue virus (DENV), Eastern Equine Encephalitis virus (EEEV), Epizootic hemorrhagic disease virus (EHDV), Guaroa virus (GROV), Hart Park virus (HPV), Ilheus virus (ILHV), Irituia virus (IRIV), Israel Turkey Meningoencephalitis virus (ITV), Japanaut virus (JAPV), Joinjakaka (JOIV), Japanese encephalitis virus (JBEV), Ketapang virus (KETV), Kunjin virus (KUNV), La Crosse virus (LACV), Mayaro virus (MAYV), Marburg virus (MBGV), Marco virus (MCOV), Melao virus (MELV), Marituba virus (MTBV), Mount Elgon bat virus (MEBV), Mucambo virus (MUCV), Murray Valley Encephalitis virus (MVEV), Navarro virus (NAVV), Nepuyo virus (NEPV), Nola virus (NOLV), Ntaya virus (NTAV), Oriboca virus (ORIV), Orungo virus (ORUV), Restan virus (RESV), Rift Valley fever virus (RVFV), Semliki Forest virus (SFV), Sindbis virus (SINV), Tahyna virus(TAHV), Tsuruse virus (TSUV), Tyuleniy virus (TYUV), Venezuelan equine encephalitis virus (VEEV), Vesicular stomatitis virus (Indiana serotype), Warrego virus (WARV), West Nile virus (WNV), Wesselsbron virus (WSLV), Yaounde virus (YAOV), Yellow fever virus (YFV), Zegla virus (ZEGV), Zika virus, as well as Plasmodium gallinaceum and Plasmodium lophurae.
This mosquito also mechanically transmits some veterinary diseases. In 1952 Fenner et al., found it transmitting the myxoma virus between rabbits[28] and in 2001 Chihota et al., the lumpy skin disease virus between cattle.[28][29]
The yellow fever mosquito can contribute to the spread of reticular cell sarcoma among Syrian hamsters.[30]
Bite prevention methods
[edit]The Centers for Disease Control and Prevention traveler's page on preventing dengue fever suggests using mosquito repellents that contain DEET (N, N-diethylmetatoluamide, 20% to 30%). It also suggests:
- Although Aedes aegypti mosquitoes most commonly feed at dusk and dawn, indoors, in shady areas, or when the weather is cloudy, "they can bite and spread infection all year long and at any time of day."[31][32]
- Once a week, scrub off eggs sticking to wet containers, seal or discard them. The mosquitoes prefer to breed in areas of stagnant water, such as flower vases, uncovered barrels, buckets, and discarded tires, but the most dangerous areas are wet shower floors and toilet tanks, as they allow the mosquitos to breed in the residence. Research has shown that certain chemicals emanating from bacteria in water containers stimulate the female mosquitoes to lay their eggs. They are particularly motivated to lay eggs in water containers that have the correct amounts of specific fatty acids associated with bacteria involved in the degradation of leaves and other organic matter in water. The chemicals associated with the microbial stew are far more stimulating to discerning female mosquitoes than plain or filtered water in which the bacteria once lived.[33]
- Wear long-sleeved clothing and long pants when outdoors during the day and evening.
- Use mosquito netting over the bed if the bedroom is not air conditioned or screened, and for additional protection, treat the mosquito netting with the insecticide permethrin.
Insect repellents containing DEET (particularly concentrated products) or p-menthane-3,8-diol (from lemon eucalyptus) were effective in repelling Ae. aegypti mosquitoes, while others were less effective or ineffective in a scientific study.[34] The Centers for Disease Control and Prevention article on "Protection against Mosquitoes, Ticks, & Other Arthropods" notes that "Studies suggest that concentrations of DEET above approximately 50% do not offer a marked increase in protection time against mosquitoes; DEET efficacy tends to plateau at a concentration of approximately 50%".[35] Other insect repellents recommended by the CDC include Picaridin (KBR 3023/icaridin), IR3535, and 2-undecanone.[36]
Population control efforts
[edit]Insecticides
[edit]Pyrethroids are commonly used.[37] This widespread use of pyrethroids and DDT has caused Knockdown resistance (kdr) mutations. Almost no research has been done on the fitness implications. studies by Kumar et al., 2009 on deltamethrin in India, Plernsub et al., 2013 on permethrin in Thailand, by Jaramillo-O et al., 2014 on λ-cyhalothrin in Colombia, by Alvarez-Gonzalez et al., 2017 on deltamethrin in Venezuela, are all substantially confounded. As of 2019, understanding of selective pressure under withdrawal of insecticide is hence limited.[37]
Genetic modification
[edit]Ae. aegypti has been genetically modified to suppress its own species in an approach similar to the sterile insect technique, thereby reducing the risk of disease. The mosquitoes, known as OX513A, were developed by Oxitec, a spinout of Oxford University. Field trials in the Cayman Islands,[38] in Juazeiro,[39][40] Brazil,[38] by Carvalho et al., 2015,[39][40] and in Panama[38] by Neira et al., 2014[39] have shown that the OX513A mosquitoes reduced the target mosquito populations by more than 90%. This mosquito suppression effect is achieved by a self-limiting gene that prevents the offspring from surviving. Male modified mosquitoes, which do not bite or spread disease, are released to mate with the pest females. Their offspring inherit the self-limiting gene and die before reaching adulthood—before they can reproduce or spread disease. The OX513A mosquitoes and their offspring also carry a fluorescent marker for simple monitoring. To produce more OX513A mosquitoes for control projects, the self-limiting gene is switched off (using the Tet-Off system) in the mosquito production facility using an antidote (the antibiotic tetracycline), allowing the mosquitoes to reproduce naturally. In the environment, the antidote is unavailable to rescue mosquito reproduction, so the pest population is suppressed.[41]
The mosquito control effect is nontoxic and species-specific, as the OX513A mosquitoes are Ae. aegypti and only breed with Ae. aegypti. The result of the self-limiting approach is that the released insects and their offspring die and do not persist in the environment.[42][43]
In Brazil, the modified mosquitoes were approved by the National Biosecurity Technical Commission for releases throughout the country. Insects were released into the wild populations of Brazil, Malaysia, and the Cayman Islands in 2012.[44][45] In July 2015, the city of Piracicaba, São Paulo, started releasing the OX513A mosquitoes.[46][47] In 2015, the UK House of Lords called on the government to support more work on genetically modified insects in the interest of global health.[48] In 2016, the United States Food and Drug Administration granted preliminary approval for the use of modified mosquitoes to prevent the spread of the Zika virus.[49]
Another proposed method consists in using radiation to sterilize male larvae so that when they mate, they produce no progeny.[50] Male mosquitoes do not bite or spread disease.
Using CRISPR/Cas9 based genome editing to engineer the genome of Aedes aegypti genes like ECFP (enhanced cyan fluorescent protein), Nix (male-determining factor gene), Aaeg-wtrw (Ae. aegypti water witch locus), Kmo (kynurenine 3-monoxygenase), loqs (loquacious), r2d2 (r2d2 protein), ku70 (ku heterodimer protein gene) and lig4 (ligase4) were targeted to modify the genome of Aedes aegypti. The new mutant will become incapable of pathogen transmission or result in population control.[51]
Infection with Wolbachia
[edit]In 2016 research into the use of a bacterium called Wolbachia as a method of biocontrol was published showing that invasion of Ae. aegypti by the endosymbiotic bacteria allows mosquitos to be resistant to certain arboviruses such as dengue fever and Zika virus strains currently circulating.[52][53][54] In 2017 Alphabet, Inc. started the Debug Project to infect males of this species with Wolbachia bacteria, interrupting the reproductive cycle of these animals.[55]
Fungus infection
[edit]Fungal species Erynia conica (from the family Entomophthoraceae) infects (and kills) two types of mosquitos: Aedes aegypti and Culex restuans. Studies on the fungus have been carried out on its potiential use as a biological control of the mosquitos.[56]
Taxonomy
[edit]The species was first named (as Culex aegypti) in 1757 by Fredric Hasselquist in his treatise Iter Palaestinum.[57] Hasselquist was provided with the names and descriptions by his mentor, Carl Linnaeus. This work was later translated into German and published in 1762 as Reise nach Palästina.[58]

To stabilise the nomenclature, a petition to the International Commission on Zoological Nomenclature was made by P. F. Mattingly, Alan Stone, and Kenneth L. Knight in 1962.[59] It also transpired that, although the name Aedes aegypti was universally used for the yellow fever mosquito, Linnaeus had actually described a species now known as Aedes (Ochlerotatus) caspius.[59] In 1964, the commission ruled in favour of the proposal, validating Linnaeus' name, and transferring it to the species for which it was in general use.[60]
The yellow fever mosquito belongs to the tribe Aedini of the dipteran family Culicidae and to the genus Aedes and subgenus Stegomyia. According to one recent analysis, the subgenus Stegomyia of the genus Aedes should be raised to the level of genus.[61] The proposed name change has been ignored by most scientists;[62] at least one scientific journal, the Journal of Medical Entomology, has officially encouraged authors dealing with aedile mosquitoes to continue to use the traditional names, unless they have particular reasons for not doing so.[63] The generic name comes from the Ancient Greek ἀηδής, aēdēs, meaning "unpleasant"[64] or "odious".
Subspecies
[edit]Two subspecies are commonly recognized:
This classification is complicated by the results of Gloria-Soria et al., 2016. Although confirming the existence of these two major subspecies, Gloria-Sora et al. finds greater worldwide diversity than previously recognized and a large number of distinct populations separated by various geographic factors.[2][3] Aedes aegypti formosus is found in natural habitats such as forests, while Aedes aegypti aegypti has adapted to urban domestic habitats. [65]
See also
[edit]References
[edit]- ^ a b Neal L. Evenhuis; Samuel M. Gon III (2007). "22. Family Culicidae" (PDF). In Neal L. Evenhuis (ed.). Catalog of the Diptera of the Australasian and Oceanian Regions. Bishop Museum. pp. 191–218. Retrieved February 4, 2012.
- ^ a b c d Souza-Neto, Jayme A.; Powell, Jeffrey R.; Bonizzoni, Mariangela (2019). "Aedes aegypti vector competence studies: A review". Infection, Genetics and Evolution. 67. Elsevier: 191–209. Bibcode:2019InfGE..67..191S. doi:10.1016/j.meegid.2018.11.009. ISSN 1567-1348. PMC 8135908. PMID 30465912.
- ^ a b c d Weetman, David; Kamgang, Basile; Badolo, Athanase; Moyes, Catherine L.; Shearer, Freya M.; Coulibaly, Mamadou; Pinto, João; Lambrechts, Louis; McCall, Philip J. (2018-01-28). "Aedes Mosquitoes and Aedes-Borne Arboviruses in Africa: Current and Future Threats". International Journal of Environmental Research and Public Health. 15 (2). MDPI: 220. doi:10.3390/ijerph15020220. ISSN 1660-4601. PMC 5858289. PMID 29382107.
- ^ Catherine Zettel and Phillip Kaufman (March 2019). "Aedes aegypti (Linnaeus)". entnemdept.ufl.edu. Retrieved 2022-03-12.
- ^ a b Roland Mortimer (nd). "Micscape Microscopy and Microscope Magazine". www.microscopy-uk.org.uk. Retrieved 2022-03-12.
- ^ Geier, Martin; Bosch, Oliver J.; Boeckh, Jürgen (1 December 1999). "Ammonia as an Attractive Component of Host Odour for the Yellow Fever Mosquito, Aedes aegypti". Chemical Senses. 24 (6): 647–653. doi:10.1093/chemse/24.6.647. ISSN 0379-864X. PMID 10587497.
- ^ Ghaninia, Majid; Majeed, Shahid; Dekker, Teun; Hill, Sharon R.; Ignell, Rickard (30 December 2019). "Hold your breath – Differential behavioral and sensory acuity of mosquitoes to acetone and carbon dioxide". PLOS ONE. 14 (12): e0226815. Bibcode:2019PLoSO..1426815G. doi:10.1371/journal.pone.0226815. ISSN 1932-6203. PMC 6936819. PMID 31887129.
- ^ Bohbot, Jonathan D.; Durand, Nicolas F.; Vinyard, Bryan T.; Dickens, Joseph C. (2013). "Functional Development of the Octenol Response in Aedes aegypti". Frontiers in Physiology. 4: 39. doi:10.3389/fphys.2013.00039. PMC 3590643. PMID 23471139.
- ^ Dennis O'Brien (March 9, 2010). "ARS Study Provides a Better Understanding of How Mosquitoes Find a Host". U.S. Department of Agriculture. Archived from the original on 8 October 2010. Retrieved 2010-08-27.
- ^ McBride, Carolyn S.; Baier, Felix; Omondi, Aman B.; Spitzer, Sarabeth A.; Lutomiah, Joel; Sang, Rosemary; Ignell, Rickard; Vosshall, Leslie B. (12 November 2014). "Evolution of mosquito preference for humans linked to an odorant receptor". Nature. 515 (7526): 222–227. Bibcode:2014Natur.515..222M. doi:10.1038/nature13964. PMC 4286346. PMID 25391959.
- ^ Catherine Zettel; Phillip Kaufman. "Yellow fever mosquito Aedes aegypti". University of Florida, Institute of Food and Agricultural Sciences. Retrieved 2010-08-27.
- ^ Roland Mortimer. "Aedes aegypti and dengue fever". Onview.net Ltd, Microscopy-UK. Retrieved 2010-08-27.
- ^ Carpenter, Simon; Mellor, Philip S.; Fall, Assane G.; Garros, Claire; Venter, Gert J. (2017-01-31). "African Horse Sickness Virus: History, Transmission, and Current Status". Annual Review of Entomology. 62 (1). Annual Reviews: 343–358. doi:10.1146/annurev-ento-031616-035010. ISSN 0066-4170. PMID 28141961.
- ^ Takken, Willem; Verhulst, Niels O. (2013-01-07). "Host Preferences of Blood-Feeding Mosquitoes". Annual Review of Entomology. 58 (1). Annual Reviews: 433–453. doi:10.1146/annurev-ento-120811-153618. ISSN 0066-4170. PMID 23020619.
- ^ Laurence Mousson; Catherine Dauga; Thomas Garrigues; Francis Schaffner; Marie Vazeille; Anna-Bella Failloux (August 2005). "Phylogeography of Aedes (Stegomyia) aegypti (L.) and Aedes (Stegomyia) albopictus (Skuse) (Diptera: Culicidae) based on mitochondrial DNA variations". Genetics Research. 86 (1): 1–11. doi:10.1017/S0016672305007627. PMID 16181519.
- ^ Eisen, L.; Moore, C. G. (2013). "Aedes (Stegomyia) aegypti in the Continental United States: A Vector at the Cool Margin of Its Geographic Range". Journal of Medical Entomology. 50 (3): 467–478. doi:10.1603/ME12245. PMID 23802440. S2CID 16922806.
- ^ M. Womack (1993). "The yellow fever mosquito, Aedes aegypti". Wing Beats. 5 (4): 4.
- ^ "Aedes aegypti". European Centre for Disease Prevention and Control. 9 June 2017.
- ^ "Mosquitoes capable of carrying Zika virus found in Washington, D.C." University of Notre Dame. 2016.
- ^ Climate Crisis Could Expose Half a Billion More People to Tropical Mosquito-Borne Diseases by 2050, Common Dreams, Jessica Corbett, March 29, 2019. Retrieved March 31, 2019.
- ^ Warchot, Allan; Whelan, Peter; Brown, John; Vincent, Tony; Carter, Jane; Kurucz, Nina (2020). "The Removal of Subterranean Stormwater Drain Sumps as Mosquito Breeding Sites in Darwin, Australia". Tropical Medicine and Infectious Disease. 5 (1): 9. doi:10.3390/tropicalmed5010009. PMC 7157592. PMID 31936813.
- ^ Kraemer, Moritz U. G.; Reiner, Robert C.; Brady, Oliver J.; Messina, Jane P.; Gilbert, Marius; Pigott, David M.; Yi, Dingdong; Johnson, Kimberly; Earl, Lucas; Marczak, Laurie B.; Shirude, Shreya; Davis Weaver, Nicole; Bisanzio, Donal; Perkins, T. Alex; Lai, Shengjie; Lu, Xin; Jones, Peter; Coelho, Giovanini E.; Carvalho, Roberta G.; Van Bortel, Wim; Marsboom, Cedric; Hendrickx, Guy; Schaffner, Francis; Moore, Chester G.; Nax, Heinrich H.; Bengtsson, Linus; Wetter, Erik; Tatem, Andrew J.; Brownstein, John S.; Smith, David L.; Lambrechts, Louis; Cauchemez, Simon; Linard, Catherine; Faria, Nuno R.; Pybus, Oliver G.; Scott, Thomas W.; Liu, Qiyong; Yu, Hongjie; Wint, G. R. William; Hay, Simon I.; Golding, Nick (4 March 2019). "Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus". Nature Microbiology. 4 (5): 854–863. doi:10.1038/s41564-019-0376-y. PMC 6522366. PMID 30833735.
- ^ a b Jeannin, Charles; Perrin, Yvon; Cornelie, Sylvie; Gloria-Soria, Andrea; Gauchet, Jean-Daniel; Robert, Vincent (2022). "An alien in Marseille: investigations on a single Aedes aegypti mosquito likely introduced by a merchant ship from tropical Africa to Europe". Parasite. 29: 42. doi:10.1051/parasite/2022043. PMC 9479680. PMID 36111976. S2CID 252309456.

- ^ Heather Kowalski (May 17, 2007). "Scientists at J. Craig Venter Institute publish draft genome sequence from Aedes aegypti, mosquito responsible for yellow fever, dengue fever". J. Craig Venter Institute. Archived from the original on 2007-07-15. Retrieved 2007-05-18.
- ^ Vishvanath Nene; Jennifer R. Wortman; Daniel Lawson; Brian Haas; Chinnappa Kodira; et al. (June 2007). "Genome sequence of Aedes aegypti, a major arbovirus vector". Science. 316 (5832): 1718–1723. Bibcode:2007Sci...316.1718N. doi:10.1126/science.1138878. PMC 2868357. PMID 17510324.
- ^ Cosby, Rachel L.; Chang, Ni-Chen; Feschotte, Cédric (2019-09-01). "Host–transposon interactions: conflict, cooperation, and cooption". Genes & Development. 33 (17–18). Cold Spring Harbor Laboratory Press & The Genetics Society: 1098–1116. doi:10.1101/gad.327312.119. ISSN 0890-9369. PMC 6719617. PMID 31481535.
- ^ Walter Reed Biosystematics Unit (WRBU) (2021). "Aedes aegypti (Linnaeus, 1762)". www.wrbu.si.edu. Archived from the original on 2022-03-11. Retrieved 2022-03-12.
- ^ a b Babiuk, S.; Bowden, T. R.; Boyle, D. B.; Wallace, D. B.; Kitching, R. P. (2008). "Capripoxviruses: An Emerging Worldwide Threat to Sheep, Goats and Cattle". Transboundary and Emerging Diseases. 55 (7). Wiley: 263–272. doi:10.1111/j.1865-1682.2008.01043.x. hdl:2263/9495. ISSN 1865-1674. PMID 18774991. S2CID 20602452.
- ^ Tuppurainen, Eeva; Oura, Chris (2014). "Lumpy skin disease: an African cattle disease getting closer to the EU". Veterinary Record. 175 (12). British Veterinary Association (Wiley): 300–301. doi:10.1136/vr.g5808. ISSN 0042-4900. PMID 25256729. S2CID 10245575.
- ^ Banfield, William G.; Woke, P. A.; MacKay, C. M.; Cooper, H. L. (28 May 1965). "Mosquito Transmission of a Reticulum Cell Sarcoma of Hamsters". Science. 148 (3674): 1239–1240. Bibcode:1965Sci...148.1239B. doi:10.1126/science.148.3674.1239. PMID 14280009. S2CID 12611674.
- ^ "Travelers' Health Outbreak Notice". Centers for Disease Control and Prevention. June 2, 2010. Archived from the original on 26 August 2010. Retrieved 2010-08-27.
- ^ "Dengue Virus: Vector And Transmission". 2009-08-07. Retrieved 19 October 2012.
- ^ "Lay Your Eggs Here". Newswise, Inc. July 3, 2008. Retrieved 2010-08-27.
- ^ Rodriguez Stacy D.; Drake Lisa L.; Price David P.; Hammond John I.; Hansen Immo A. (2015). "The Efficacy of Some Commercially Available Insect Repellents for Aedes aegypti (Diptera: Culicidae) and Aedes albopictus (Diptera: Culicidae)". Journal of Insect Science. 15: 140. doi:10.1093/jisesa/iev125. PMC 4667684. PMID 26443777.
- ^ "Protection against Mosquitoes, Ticks, & Other Arthropods - Chapter 2 - 2016 Yellow Book | Travelers' Health | CDC". wwwnc.cdc.gov. Retrieved 2016-12-08.
- ^ "Prevent Tick and Mosquito Bites | Division of Vector-Borne Diseases | NCEZID | CDC". www.cdc.gov. 2019-10-07. Retrieved 2020-04-30.
- ^ a b Scott, Jeffrey G. (2019-01-07). "Life and Death at the Voltage-Sensitive Sodium Channel: Evolution in Response to Insecticide Use". Annual Review of Entomology. 64 (1). Annual Reviews: 243–257. doi:10.1146/annurev-ento-011118-112420. ISSN 0066-4170. PMID 30629893. S2CID 58667542.
- ^ a b c Kate Kelland (16 December 2015). "Lawmakers call for British trials of genetically modified insects". Reuters. Retrieved 2015-12-16.
- ^ a b c Danilo O. Carvalho; Andrew R. McKemey; Luiza Garziera; Renaud Lacroix; Christl A. Donnelly; Luke Alphey; Aldo Malavasi; Margareth L. Capurro (July 2015). "Suppression of a Field Population of Ae. aegypti in Brazil by Sustained Release of Transgenic Male Mosquitoes". PLOS Neglected Tropical Diseases. 9 (7): e0003864. doi:10.1371/journal.pntd.0003864. PMC 4489809. PMID 26135160.
- ^ a b Brady, Oliver J.; Hay, Simon I. (2020-01-07). "The". Annual Review of Entomology. 65 (1). Annual Reviews: 191–208. doi:10.1146/annurev-ento-011019-024918. ISSN 0066-4170. PMID 31594415. S2CID 203983175.
- ^ Zoe Curtis; Kelly Matzen; Marco Neira Oviedo; Derric Nimmo; Pamela Gray; Peter Winskill; Marco A. F. Locatelli; Wilson F. Jardim; Simon Warner; Luke Alphey; Camilla Beech (August 2015). "Assessment of the Impact of Potential Tetracycline Exposure on the Phenotype of Aedes aegypti OX513A: Implications for Field Use". PLOS Neglected Tropical Diseases. 9 (8): e0003999. doi:10.1371/journal.pntd.0003999. PMC 4535858. PMID 26270533.
- ^ Kevin Gorman; Josué Young; Lleysa Pineda; Ricardo Márquez; Nestor Sosa; Damaris Bernal; Rolando Torres; Yamilitzel Soto; Renaud Lacroix; Neil Naish; Paul Kaiser; Karla Tepedino; Gwilym Philips; Cecilia Kosmann; Lorenzo Cáceres (September 2015). "Short-term suppression of Aedes aegypti using genetic control does not facilitate Aedes albopictus". Pest Management Science. 72 (3): 618–628. doi:10.1002/ps.4151. PMC 5057309. PMID 26374668.
- ^ Oreenaiza Nordin; Wesley Donald; Wong Hong Ming; Teoh Guat Ney; Khairul Asuad Mohamed; Nor Azlina Abdul Halim; Peter Winskill; Azahari Abdul Hadi; Zulkamal Safi'in Muhammad; Renaud Lacroix; Sarah Scaife; Andrew Robert McKemey; Camilla Beech; Murad Shahnaz; Luke Alphey; Derric David Nimmo; Wasi Ahmed Nazni; Han Lim Lee (March 2013). "Oral Ingestion of Transgenic RIDL Ae. aegypti Larvae Has No Negative Effect on Two Predator Toxorhynchites Species". PLOS One. 8 (3): e58805. Bibcode:2013PLoSO...858805N. doi:10.1371/journal.pone.0058805. PMC 3604150. PMID 23527029.
- ^ Griffiths, Elle (January 31, 2016). "Zika outbreak 'caused by release of genetically modified mosquitoes in Brazil'". mirror.
- ^ "Can GM mosquitoes rid the world of a major killer?". the Guardian. July 14, 2012.
- ^ Justine Alford via IFLScience (25 July 2014). "Brazil To Unleash Genetically Modified Mosquitoes". Huffington Post. Retrieved 2014-07-25.
- ^ no by-line (30 April 2015). "Modified mosquitoes enter the war against dengue in São Paulo". G1. Retrieved 2015-04-30.
- ^ "Release potential of GM insects to fight disease and pests". Parliament UK. House of Lords Science and Technology Select Committee. 17 December 2015. Retrieved 2015-12-17.
- ^ "Preliminary Finding of No Significant Impact (FONSI) In Support of an Investigational Field Trial of OX513A Aedes aegypti Mosquitoes" (PDF). US FDA. March 2016. Retrieved 14 March 2016.
- ^ Tirone, Jonathan (12 February 2016). "UN Readies Nuclear Solution to Destroy the Zika Virus". Bloomberg. Retrieved 2016-02-13.
- ^ Reegan AD; Ceasar SA; Paulraj MG; Ignacimuthu S; Al-Dhabi NA (January 2017). "Current status of genome editing in vector mosquitoes: A review". BioScience Trends. 10 (6): 424–432. doi:10.5582/bst.2016.01180. PMID 27990003.
- ^ Dutra, HL; Rocha, MN; Dias, FB; Mansur, SB; Caragata, EP; Moreira, LA (June 8, 2016). "Wolbachia Blocks Currently Circulating Zika Virus Isolates in Brazilian Aedes aegypti Mosquitoes". Cell Host & Microbe. 19 (6): 771–774. doi:10.1016/j.chom.2016.04.021. PMC 4906366. PMID 27156023 – via PMC.
- ^ Hancock, Penelope A.; White, Vanessa L.; Callahan, Ashley G.; Godfray, Charles H. J.; Hoffmann, Ary A.; Ritchie, Scott A.; Clough, Yann (June 2016). "Density-dependent population dynamics in Aedes aegypti slow the spread of wMel Wolbachia". Journal of Applied Ecology. 53 (3): 785–793. Bibcode:2016JApEc..53..785H. doi:10.1111/1365-2664.12620. hdl:10044/1/103425.
- ^ Utarini, Adi; Indriani, Citra; Ahmad, Riris A.; Tantowijoyo, Warsito; Arguni, Eggi; Ansari, M. Ridwan; Supriyati, Endah; Wardana, D. Satria; Meitika, Yeti; Ernesia, Inggrid; Nurhayati, Indah; Prabowo, Equatori; Andari, Bekti; Green, Benjamin R.; Hodgson, Lauren; Cutcher, Zoe; Rancès, Edwige; Ryan, Peter A.; O'Neill, Scott L.; Dufault, Suzanne M.; Tanamas, Stephanie K.; Jewell, Nicholas P.; Anders, Katherine L.; Simmons, Cameron P. (10 June 2021). "Efficacy of Wolbachia-Infected Mosquito Deployments for the Control of Dengue". New England Journal of Medicine. 384 (23): 2177–2186. doi:10.1056/NEJMoa2030243. PMC 8103655. PMID 34107180.
- ^ "Let's Stop Bad Bugs With Good Bugs". De Bug Project. Verily Life Sciences LLC. Retrieved 16 July 2017.
- ^ Cuebas-Incle, E. L. (December 1992). "Infection of adult mosquitoes by the entomopathogenic fungus Erynia conica (Entomophthorales: Entomophthoraceae)". J Am Mosq Control Assoc. 8 (4): 367–71. PMID 1474381.
- ^ Hasselquist, Fredrik, Carl von Linné (1757): Iter Palæstinum, Eller, Resa til Heliga Landet, Förrättad Infrån år 1749 til 1752
- ^ Hasselquist, Friedrich (August 4, 1762). "Reise nach Palästina in den Jahren von 1749 bis 1752". Koppe – via Google Books.
- ^ a b P. F. Mattingly; Alan Stone; Kenneth L. Knight (1962). "Culex aegypti Linnaeus, 1762 (Insecta, Diptera); proposed validation and interpretation under the plenary powers of the species so named. Z.N.(S.) 1216" (PDF). Bulletin of Zoological Nomenclature. 19 (4): 208–219. Archived from the original (PDF) on 2012-03-01.
- ^ International Commission on Zoological Nomenclature (1964). "Culex aegypti Linnaeus, 1762 (Insecta, Diptera): validated and interpreted under the plenary powers". Bulletin of Zoological Nomenclature. 21 (4): 246–248.
- ^ John F. Reinert; Ralph E. Harbach; Ian J. Kitching (2004). "Phylogeny and classification of Aedini (Diptera: Culicidae), based on morphological characters of all life stages". Zoological Journal of the Linnean Society. 142 (3): 289–368. doi:10.1111/j.1096-3642.2004.00144.x.
- ^ Andrew Polaszek (January 2006). "Two words colliding: resistance to changes in the scientific names of animals – Aedes vs Stegomyia". Trends in Parasitology. 22 (1): 8–9. doi:10.1016/j.pt.2005.11.003. PMID 16300998.
- ^ "Journal of Medical Entomology Policy on Names of Aedine Mosquito Genera and Subgenera". Entomological Society of America. Archived from the original on August 9, 2017. Retrieved August 31, 2011.
- ^ "Etymologia: Aedes aegypti". Emerg Infect Dis. 22 (10): 1807. October 2016. doi:10.3201/eid2210.ET2210. PMC 5038420.
- ^ "Aedes aegypti - Factsheet for experts". www.ecdc.europa.eu. 2017-06-09. Retrieved 2024-10-18.
External links
[edit]- Aedes aegypti on the entomology Institute of Food and Agricultural Sciences Featured Creatures Web site University of Florida.
- United States CDC page on dengue fever containing information on prevalence of Aedes aegypti worldwide and past efforts to eradicate it
- The ecology and biology of Aedes aegypti (L.) and Aedes albopictusand the resistance of Aedes albopictus against organophosphates in Penang, Malaysia M.S. thesis.
- Aedes aegypti Community Eradication using copepods. The Monte Verde Story (Honduras)
