Absorption refrigerator: Difference between revisions
m Reverting possible vandalism by 203.25.141.5 to version by ScottMHoward. False positive? Report it. Thanks, ClueBot. (641796) (Bot) |
|||
| Line 26: | Line 26: | ||
A single-pressure absorption refrigerator uses three substances: [[ammonia]], [[hydrogen]] gas, and water, whereas large industrial units generally use only two: a refrigerant such as ammonia, and an absorbent such as water (with an expansion valve and pump, not described here). Normally, ammonia is a gas at room temperature (with a boiling point of -33 °C), but the system is pressurized to the point that the ammonia is a liquid at room temperature. |
A single-pressure absorption refrigerator uses three substances: [[ammonia]], [[hydrogen]] gas, and water, whereas large industrial units generally use only two: a refrigerant such as ammonia, and an absorbent such as water (with an expansion valve and pump, not described here). Normally, ammonia is a gas at room temperature (with a boiling point of -33 °C), but the system is pressurized to the point that the ammonia is a liquid at room temperature. |
||
The cooling cycle starts at the evaporator, where liquefied [[anhydrous]] ammonia enters. ('Anhydrous' means there is no water in the ammonia, which is critical for exploiting its sub-zero boiling point.) The "evaporator" contains another gas (in this case, hydrogen), whose presence lowers the [[partial pressure]] of the ammonia in that part of the system. The total pressure in the system is still the same, but now not all of the pressure is being exerted by ammonia, as much of it is due to the pressure of the hydrogen. Ammonia doesn't react with hydrogen - the hydrogen is there solely to take up space - creating a void that still has the same pressure as the rest of the system, but not in the form of ammonia. Per [[Dalton's law]], the ammonia behaves only in response to the [[Partial pressure|proportion of the pressure]] represented by the ammonia, as if there was a vacuum and the hydrogen wasn't there. Because a substance's boiling point changes with pressure, the lowered partial pressure of ammonia changes the ammonia's boiling point, bringing it low enough that it can now boil below room temperature, as though it weren't under the pressure of the system in the first place. When it boils, it takes some heat away with it from the evaporator - which produces the "cold" desired in the refrigerator. |
The cooling cycle starts at the evaporator, where liquefied [[anhydrous]] ammonia enters. ('Anhydrous' means there is no water in the ammonia, which is critical for exploiting its sub-zero boiling point.) The "evaporator" contains another gas (in this case, hydrogen), whose presence lowers the [[partial pressure]] of the ammonia in that part of the system. The total pressure in the system is still the same, but now not all of the pressure is being exerted by ammonia, as much of it is due to the pressure of the hydrogen. Ammonia doesn't react with hydrogen - the hydrogen is there solely to take up space - creating a void that still has the same pressure as the rest of the system, but not in the form of ammonia. Per [[Dalton's law]], the ammonia behaves only in response to the [[Partial pressure|proportion of the pressure]] represented by the ammonia, as if there was a vacuum and the hydrogen wasn't there. Because a substance's boiling point changes with pressure, the lowered partial pressure of ammonia changes the ammonia's boiling point, bringing it low enough that it can now boil below room temperature, as though it weren't under the pressure of the system in the first place. When it boils, it takes some heat away with it from the evaporator - which produces the "cold" desired in the refrigerator. va jay jay |
||
The next step is separating the gaseous ammonia-hydrogen mixture. Separation from the hydrogen is simple, and this is where the "absorber" comes in. Ammonia readily mixes with water, and hydrogen does not. The absorber is simply a downhill run of tubes in which the mixture of gases counterflows upwards in contact with water trickling down. At the top of the flow, with most of the ammonia gone into solution there, the gas is mainly hydrogen, free to return to the evaporator, while at the bottom of the flow, the dissolved AND gaseous ammonia concentrations are highest respectively, which is in accordance with the principle of a counterflow absorber, and now the solution is ready for entry to the boiler. |
The next step is separating the gaseous ammonia-hydrogen mixture. Separation from the hydrogen is simple, and this is where the "absorber" comes in. Ammonia readily mixes with water, and hydrogen does not. The absorber is simply a downhill run of tubes in which the mixture of gases counterflows upwards in contact with water trickling down. At the top of the flow, with most of the ammonia gone into solution there, the gas is mainly hydrogen, free to return to the evaporator, while at the bottom of the flow, the dissolved AND gaseous ammonia concentrations are highest respectively, which is in accordance with the principle of a counterflow absorber, and now the solution is ready for entry to the boiler. |
||
Revision as of 03:48, 9 July 2010
The absorption refrigerator is a refrigerator that uses a heat source (e.g., solar, kerosene-fueled flame) to provide the energy needed to drive the cooling system. Absorption refrigerators are a popular alternative to regular compressor refrigerators where electricity is unreliable, costly, or unavailable, where noise from the compressor is problematic, or where surplus heat is available (e.g., from turbine exhausts or industrial processes). Absorption refrigerators powered by heat from the combustion of liquefied petroleum gas are often used for food storage in recreational vehicles.
Both absorption and compressor refrigerators use a refrigerant with a very low (less than 0 °F (−18 °C)*) boiling point. In both types, when this refrigerant evaporates or boils, it takes some heat away with it, providing the cooling effect. The main difference between the two types is the way the refrigerant is changed from a gas back into a liquid so that the cycle can repeat. A compressor refrigerator uses an electrically-powered compressor to increase the pressure on the gas, and then condenses the hot high pressure gas back to a liquid by heat exchange with a coolant (usually air). Once the high pressure gas has cooled, it passes through a pressure release valve which drops the refrigerant temperature to below freezing. An absorption refrigerator changes the gas back into a liquid using a different method that needs only heat, and has no moving parts. The other difference between the two types is the refrigerant used. Compressor refrigerators typically use an HCFC, while absorption refrigerators typically use ammonia.
The standard for the absorption refrigerator is given by the ANSI/AHRI Standard 560-2000[1].
Principles
Absorptive refrigeration uses a source of heat to provide the energy needed to drive the cooling process. The most common use is in commercial climate control and cooling of machinery. Absorptive refrigeration is also used to air-condition buildings using the waste heat from a gas turbine or water heater. The process is very efficient, since the gas turbine produces electricity, hot water and air-conditioning (see Trigeneration).
The basic thermodynamic process is not a conventional thermodynamic cooling process based on Charles' law. Instead, it is based on evaporation carrying heat, in the form of faster-moving (hotter) molecules, from one material to another material that preferentially absorbs hot molecules.
A familiar example is human sweating. The water in sweat evaporates and is "absorbed" into air, carrying away heat from the body. However, absorptive refrigerators differ in that they regenerate their coolants in a closed cycle, while people need to keep replacing their lost water (evaporated sweat) through drinking.

The classic gas absorption refrigerator sends liquid ammonia into a hydrogen gas. The liquid ammonia evaporates in the presence of hydrogen gas, providing the cooling. The now-gaseous ammonia is sent into a container holding water, which absorbs the ammonia. The water-ammonia solution is then directed past a heater, which boils ammonia gas out of the water-ammonia solution. The ammonia gas is then condensed into a liquid. The liquid ammonia is then sent back through the hydrogen gas, completing the cycle.
A similar system, common in large commercial plants, uses a solution of lithium bromide salt and water. Water under low pressure is evaporated from the coils that are being chilled. The water is absorbed by a lithium bromide/water solution. The water is driven off the lithium bromide solution using heat.
Absorption chillers are a different approach to achieving air conditioning and process cooling. Absorption chillers are driven by hot water rather than from large amounts of electricity like conventional air conditioners. This hot water may come from any number of industrial sources. There are no CFCs or freons, no lithium bromide, and no ammonia. By replacing the corrosive salt desiccant with a benign silica gel, adsorption chillers significantly reduce maintenance and upkeep costs
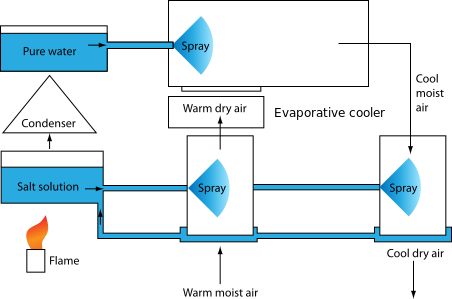
Another variant uses air, water, and a salt water solution. As shown in the figure below, warm moist air is passed through a sprayed solution of salt water. The spray lowers the humidity. The less humid warm air is then passed through an evaporative cooler which cools and re-humidifies. Humidity is removed from the cooled air with another spray of salt solution. The salt solution is regenerated by heating it under low pressure, causing water to evaporate. The water evaporated from the salt solution is re-condensed, and rerouted back to the evaporative cooler.
Process
A single-pressure absorption refrigerator uses three substances: ammonia, hydrogen gas, and water, whereas large industrial units generally use only two: a refrigerant such as ammonia, and an absorbent such as water (with an expansion valve and pump, not described here). Normally, ammonia is a gas at room temperature (with a boiling point of -33 °C), but the system is pressurized to the point that the ammonia is a liquid at room temperature.
The cooling cycle starts at the evaporator, where liquefied anhydrous ammonia enters. ('Anhydrous' means there is no water in the ammonia, which is critical for exploiting its sub-zero boiling point.) The "evaporator" contains another gas (in this case, hydrogen), whose presence lowers the partial pressure of the ammonia in that part of the system. The total pressure in the system is still the same, but now not all of the pressure is being exerted by ammonia, as much of it is due to the pressure of the hydrogen. Ammonia doesn't react with hydrogen - the hydrogen is there solely to take up space - creating a void that still has the same pressure as the rest of the system, but not in the form of ammonia. Per Dalton's law, the ammonia behaves only in response to the proportion of the pressure represented by the ammonia, as if there was a vacuum and the hydrogen wasn't there. Because a substance's boiling point changes with pressure, the lowered partial pressure of ammonia changes the ammonia's boiling point, bringing it low enough that it can now boil below room temperature, as though it weren't under the pressure of the system in the first place. When it boils, it takes some heat away with it from the evaporator - which produces the "cold" desired in the refrigerator. va jay jay
The next step is separating the gaseous ammonia-hydrogen mixture. Separation from the hydrogen is simple, and this is where the "absorber" comes in. Ammonia readily mixes with water, and hydrogen does not. The absorber is simply a downhill run of tubes in which the mixture of gases counterflows upwards in contact with water trickling down. At the top of the flow, with most of the ammonia gone into solution there, the gas is mainly hydrogen, free to return to the evaporator, while at the bottom of the flow, the dissolved AND gaseous ammonia concentrations are highest respectively, which is in accordance with the principle of a counterflow absorber, and now the solution is ready for entry to the boiler.
At this point in the cycle, the ammonia-water solution is not usable for refrigeration, as the mixture won't boil at a low enough temperature to be usable as a refrigerant. This is where the heat from the flame comes in. When the right amount of heat is applied to the mixture, the ammonia bubbles out. This phase is called the "generator" phase. The ammonia isn't quite dry yet - the bubbles contain gas but they're made of water, so the pipe twists and turns and contains a few minor obstacles that pop the bubbles so the gas can move on. The water that results from the bubbles isn't bad - it takes care of another need, and that is the circulation of water through the previous absorption step. Because that water has risen a bit while it was bubbling upwards, the flow of that water falling back down due to gravity can be used for this purpose. The maze that makes the ammonia gas go one way and the bubble water go the other is called the "separator".
The next step is the condenser. The condenser is a sort of heat sink or heat exchanger that cools the hot ammonia gas back down to room temperature. Because of the pressure and the purity of the gas (there is no hydrogen or water here), the ammonia condenses back into a liquid, and at that point, it's suitable as a refrigerant and the cycle starts over again.
History
Absorption cooling was invented by the French scientist Ferdinand Carré in 1858.[2] The original design used water and sulfuric acid.
In 1922 Baltzar von Platen and Carl Munters, while they were still students at the Royal Institute of Technology in Stockholm, Sweden, enhanced the principle with a 3 fluids configuration. This "Platen-Munters" design can operate without a pump.
Commercial production began in 1923 by the newly formed company AB Arctic, which was bought by Electrolux in 1925. In the 60s the absorption refrigeration sees a renaissance due to the substantial demand for refrigerators for caravans. AB Electrolux establishes a subsidiary in the U.S. – Dometic Sales Corporation. The company markets refrigerators for caravans and other recreation equipment under the Dometic brand. In 2001 Electrolux sells most of its Leisure Products product line to the venture-capital company EQT. The Dometic Group is created.
In 1926 Albert Einstein and his former student Leó Szilárd proposed an alternative design known as Einstein refrigerator[3].
In 2007, Adam Grosser presented his research of a new, very small, "intermittent absorption" refrigeration system for use in third world countries at the TED Conference. The refrigerator is a small unit placed over a campfire, that can later be used to cool 3 gallons of water to just above freezing for 24 hours in a 30 degree Celsius environment.[4]
See also
- Absorption heat pump
- Einstein refrigerator
- Icyball
- Refrigerator
- Solar thermal cooling
- Timeline of low-temperature technology
References
- ^ http://www.ahrinet.org/Content/FindaStandard_218.aspx?Listing_PK=150
- ^ Eric Granryd & Björn Palm, Refrigerating engineering, Stockholm Royal Institute of Technology, 2005, see chap. 4-3
- ^ "US Patent 1781541".
- ^ "Adam Grosser and his sustainable fridge". TED. Retrieved 2010-04-18.
External links
- ANSI/AHRI Standard 560-2000.
- Absorption Heat Pumps (EERE).
- Arizona Energy Explanation with diagrams
- Design Analysis of the Einstein Refrigeration Cycle, Andrew Delano (1998). Retrieved September 13, 2005.
- How It Works, Details about the absorption system. Retrieved June 26, 2009.
- Ohio State University Center of Excellence in Absorption Technology: Theory of Heat Pump Operation
- Air Conditioning Thermodynamics, published by the California EPA, Air Resources Board
- Thermally-Activated Machines Refrigeration Cycle: Northeast CHP Application Center at the University of Massachusetts Amherst and Pace University