2-Naphthol

| |

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Naphthalen-2-ol | |
| Other names
2-Hydroxynaphthalene; 2-Naphthalenol; beta-Naphthol; Naphth-2-ol
| |
| Identifiers | |
3D model (JSmol)
|
|
| 742134 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.712 |
| EC Number |
|
| 27395 | |
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 3077 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H8O | |
| Molar mass | 144.173 g·mol−1 |
| Appearance | Colorless crystalline solid |
| Density | 1.280 g/cm3 |
| Melting point | 121 to 123 °C (250 to 253 °F; 394 to 396 K) |
| Boiling point | 285 °C (545 °F; 558 K) |
| 0.74 g/L | |
| Acidity (pKa) | 9.51 |
| -98.25·10−6 cm3/mol | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Harmful when inhaled or swallowed; dangerous to environment, esp. aquatic organisms.[1] |
| GHS labelling: | |
 
| |
| Warning | |
| H302, H332, H400 | |
| P261, P264, P270, P271, P273, P301+P312, P304+P312, P304+P340, P312, P330, P391, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 161 °C (322 °F; 434 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
2-Naphthol, or β-naphthol, is a fluorescent colorless (or occasionally yellow) crystalline solid with the formula C10H7OH. It is an isomer of 1-naphthol, differing by the location of the hydroxyl group on the naphthalene ring. The naphthols are naphthalene homologues of phenol, but more reactive. Both isomers are soluble in simple alcohols, ethers, and chloroform. 2-Naphthol is a widely used intermediate for the production of dyes and other compounds.
Production
[edit]Traditionally, 2-naphthol is produced by a two-step process that begins with the sulfonation of naphthalene in sulfuric acid:[2]
- C10H8 + H2SO4 → C10H7SO3H + H2O
The sulfonic acid group is then cleaved in molten sodium hydroxide:
- C10H7(SO3H) + 3 NaOH → C10H7ONa + Na2SO3 + 2 H2O
Neutralization of the product with acid gives 2-naphthol.
2-Naphthol can also be produced by a method analogous to the cumene process.[2]
2-Naphthol-derived dyes
[edit]The Sudan dyes are popular dyes noted for being soluble in organic solvents. Several of the Sudan dyes are derived from 2-naphthol by coupling with diazonium salts.[3] Sudan dyes I–IV and Sudan Red G consist of arylazo-substituted naphthols.
- Selected 2-Naphthol-derived dyes
Reactions
[edit]Some reactions of 2-naphthol are explicable with reference to its tautomerism, which produces a small amount of the keto tautomer.
One consequence of this tautomerism is the Bucherer reaction, the ammonolysis of 2-naphthol to give 2-aminonaphthalene.
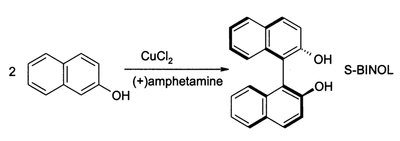
2-Naphthol can be oxidatively coupled to form BINOL, a C2-symmetric ligand popularized for use in asymmetric catalysis.
2-Naphthol converts to 2-naphthalenethiol by reaction with dimethylthiocarbamoyl chloride via the Newman–Kwart rearrangement.[4] The OH→Br conversion has been described.[5]
Electrophilic attack occurs characteristically at the 1-position as indicated by nitrosylation to give 1-nitroso-2-naphthol.[6] Bromination[7] and alkylations proceed with similar regiochemistry.[8] Ring-opening reactions have been documented.[9]
Carbonation of 2-naphthol gives 2-hydroxy-1-naphthoic acid.[2]
Safety
[edit]2-Naphthol has been described as "moderately toxic.[2]
References
[edit]- ^ a b "MSDS safety data for 2-naphthol". Archived from the original on 3 March 2011.
- ^ a b c d Booth, Gerald (2005). "Naphthalene Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_009. ISBN 978-3527306732.. full-text PDF
- ^ Booth, Gerald; Zollinger, Heinrich; McLaren, Keith; Sharples, William G.; Westwell, Alan (2000). "Dyes, General Survey". Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH. doi:10.1002/14356007.a09_073. ISBN 9783527306732.
- ^ Melvin S. Newman; Frederick W. Hetzel (1971). "Thiophenols from Phenols: 2-Naphthalenethiol". Organic Syntheses. 51: 139. doi:10.15227/orgsyn.051.0139.
- ^ J. P. Schaefer; Jerry Higgins; P. K. Shenoy (1969). "2-Bromonaphthalene". Organic Syntheses. 49: 6. doi:10.15227/orgsyn.049.0006.
- ^ Marvel, C. S.; Porter, P. K. (1922). "Nitroso-β-Naphthol". Organic Syntheses. 2: 61. doi:10.15227/orgsyn.002.0061.
- ^ C. Frederick Koelsch (1940). "6-Bromo-2-Naphthol". Organic Syntheses. 20: 18. doi:10.15227/orgsyn.020.0018.
- ^ Alfred Russell Luther B. Lockhart (1942). "2-Hydroxy-1-Naphthaldehyde". Organic Syntheses. 22: 63. doi:10.15227/orgsyn.022.0063.
- ^ G. A. Page, D. S. Tarbell (1954). "β-(o-Carboxyphenyl)propionic Acid". Organic Syntheses. 34: 8. doi:10.15227/orgsyn.034.0008.
External links
[edit]- NIST Chemistry WebBook 2-Naphthalenol
- . Encyclopædia Britannica. Vol. 19 (11th ed.). 1911. pp. 168–169.