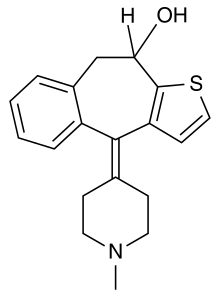
10-Hydroxyketotifen
| |
| Names | |
|---|---|
| IUPAC name
4-(1-methylpiperidin-4-ylidene)-9,10-dihydro-4H-benzo[4,5]cyclohepta[1,2-b]thiophen-10-ol
| |
| Other names
10-hydroxy-ketotifen, WR621365[1]
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
10-Hydroxyketotifen (WR621365)[1] is a biologically inactive metabolite of ketotifen. [2][3][4] Despite the mainstream scientific consensus that 10-hydroxyketotifen is a biologically inactive compound, its pharmacological properties are not very well studied outside the context of ketotifen, therefore, 10-hydroxyketotifen may still possess biological activity similarly to norketotifen, another metabolite of ketotifen.[1]
Metabolic role
[edit]Ketotifen is an antihistamine medication which metabolizes to several compounds, including 10-hydroxyketotifen. Ketotifen, like other antihistamines,[5][6] is mainly metabolized by the cytochrome P450 (CYP) enzymes, especially CYP3A4[7][8] in the liver. The CYP enzymes are responsible for the oxidation and demethylation of ketotifen, producing the major metabolites norketotifen and 10-hydroxyketotifen. Norketotifen is pharmacologically active and has a similar potency as ketotifen, while 10-hydroxyketotifen is inactive. The metabolites are then conjugated with glucuronic acid or sulfate and excreted in the urine and feces.[9][10]
The definition and measurement of biological activity of drugs can be complex: biological activity is often defined in terms of the ability of a molecule to effect a change in a biological process, which can be quantified and measured in various ways; as such, even if 10-hydroxyketotifen is currently deemed inactive, it is possible that under certain conditions or within specific biological assays, some level of activity might be observed.[1][11]
References
[edit]- ^ a b c d Milner E, Sousa J, Pybus B, Auschwitz J, Caridha D, Gardner S, et al. (2012). "Ketotifen is an antimalarial prodrug of norketotifen with blood schizonticidal and liver-stage efficacy". European Journal of Drug Metabolism and Pharmacokinetics. 37 (1): 17–22. doi:10.1007/s13318-012-0080-2. PMID 22314893.
- ^ Gunnar AK, Wright GE, Chen JL, Maioli, AT (July 3, 2002). "European Patent: optically active isomers of ketotifen and therapeutically active metabolites thereof". Archived from the original on May 2, 2024. Retrieved May 2, 2024.
- ^ Julien-Larose C, Guerret M, Lavene D, Kiechel JR (1983). "Quantification of ketotifen and its metabolites in human plasma by gas chromatography mass spectrometry". Biological Mass Spectrometry. 10 (3): 136–142. doi:10.1002/bms.1200100307. PMID 6850066.
- ^ Bersier PM, Szczepaniak W, Ren M (1992). "Direct Differential Pulse Polarographic and Adsorptive Stripping Voltammetric Assay of Ketotifen in Tablets". Archiv der Pharmazie. 325 (5): 253–259. doi:10.1002/ardp.19923250502.
- ^ Li L, Liu R, Peng C, Chen X, Li J (July 2022). "Pharmacogenomics for the efficacy and side effects of antihistamines". Exp Dermatol. 31 (7): 993–1004. doi:10.1111/exd.14602. PMID 35538735.
- ^ Merk HF (November 2001). "Standard treatment: the role of antihistamines". J Investig Dermatol Symp Proc. 6 (2): 153–6. doi:10.1046/j.0022-202x.2001.00032.x. PMID 11764306.
- ^ El-Kommos ME, El-Gizawy SM, Atia NN, Hosny NM (2015). "Analysis for commonly prescribed non-sedating antihistamines". Analytical Chemistry Research. 3: 1–12. doi:10.1016/j.ancr.2014.11.003.
- ^ Jáuregui I, Mullol J, Bartra J, del Cuvillo A, Dávila I, Montoro J, et al. (2006). "H1 antihistamines: psychomotor performance and driving". J Investig Allergol Clin Immunol. 16 (Suppl 1): 37–44. PMID 17357376.
- ^ Lieberman P, Hernandez-Trujillo V, Lieberman J, Frew AJ (2008). "Antihistamines". Clinical Immunology. pp. 1317–1329. doi:10.1016/B978-0-323-04404-2.10089-2. ISBN 978-0-323-04404-2. Archived from the original on February 24, 2024. Retrieved February 14, 2024.
- ^ "Center for drug evaluation and research. Application no. 21-066" (PDF). Archived (PDF) from the original on February 14, 2024. Retrieved February 14, 2024.
- ^ Jackson CM, Esnouf MP, Winzor DJ, Duewer DL (March 16, 2007). "Defining and measuring biological activity: Applying the principles of metrology". Accreditation and Quality Assurance. 12 (6): 283–294. doi:10.1007/s00769-006-0254-1.
